A new complaint filed by Mr. and Mrs. Duffie of Georgia joins more than two dozen Bard G2 IVC filter lawsuits that have been centralized for pretrial proceedings before Arizona U.S. District Judge David G. Campbell. Atlanta injury law firm Childers, Schlueter & Smith LLC, represents the Plaintiffs along with their stellar co-counsel: The Nations Law Firm.
Filter Fracture Led to Emergency Open Heart Surgery
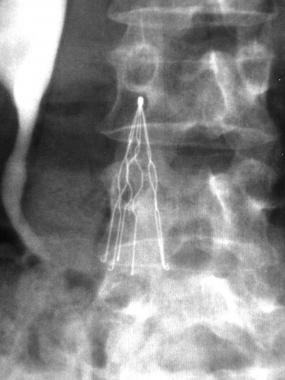
The newest IVC filter complaint alleges that Mary Duffie was forced to undergo emergency open-heart surgery in January 2014 when a leg from her Bard G2 filter broke off and perforated her heart. Much of the remainder of the IVC filter, implanted in 2008, had to be removed as well, although one leg was never found and is assumed to be still present somewhere inside her body, where it could potentially do even more harm in the future.
Mr. and Mrs. Duffie are seeking to hold C.R. Bard accountable for not recalling the IVC filter and putting lives in danger despite Bard’s knowledge of the devices failures and complications. She is also seeking compensation for permanent injury, medical expenses, lost wages and earning potential, loss of enjoyment of life, emotional trauma, and a diminished ability to handle the challenges of daily life.
What Are IVC Filters?
Inferior vena cava (IVC) filters are small medical devices shaped like spiders. They are designed to catch  blood clots and prevent them from migrating to the lungs, where they could cause a pulmonary embolism. The Bard Recovery and G2 filters were designed to be retrievable, but have been linked to numerous injuries and complications over the last few years. Common complications with IVC filters left in the body for long periods of time includes:
blood clots and prevent them from migrating to the lungs, where they could cause a pulmonary embolism. The Bard Recovery and G2 filters were designed to be retrievable, but have been linked to numerous injuries and complications over the last few years. Common complications with IVC filters left in the body for long periods of time includes:
- Recurrent PEs
- Filter migration or movement
- Tilting or breaking of the IVC filter
- IVC perforation or occlusion
According to the FDA, the majority of IVC filters are not retrieved, and their benefits have made them a frequent choice for doctors and patients, despite their perceived risk of increased fracture, embolism, and IVC wall penetration.
Contact Childers, Schlueter & Smith Today
Device manufacturers’ first responsibility is to the safety of the people using their product – it is up to them to ensure that a medical device is fit for use by the public.
When you’ve been harmed by a medical device, you need legal advice from attorneys who have experience standing up to negligent manufacturers and know how to hold them accountable for their products. We pursue nothing less than full compensation for your injuries, and that includes everything – medical expenses, physical rehabilitation, lost wages and pain and suffering.
Contact Childers, Schlueter & Smith today to speak with a lawyer who can advise you on the best course of action to take going forward. Our case evaluations are always free, and are always focused on what’s best for our clients.
Other IVC Filter Lawsuits News
An Arizona judge denied class certification for Bard IVC filter lawsuits, citing differences among plaintiffs' devices and claims. These lawsuits allege serious injuries from defective IVC filters and insufficient risk warnings.
A new study suggests IVC filters may increase pulmonary embolism risks in traumatic spine injury patients, raising concerns about their safety compared to other preventive treatments.
Studies reveal that Cook Medical’s Celect IVC filters may puncture the vena cava within two months, leading to serious complications and fueling thousands of lawsuits alleging defective design and failure to warn.
A recent study suggests IVC filters may be overused in managed care, often left in place longer than FDA guidelines recommend, raising concerns about patient safety and medical oversight.
Many IVC filters remain in patients indefinitely despite FDA recommendations for removal, raising concerns about serious complications such as fracture, migration, and vena cava perforation.
A new bellwether schedule has been issued in Bard IVC filter litigation, as thousands of lawsuits move forward alleging serious complications linked to the company’s retrievable devices.