Recent studies have concluded that over 40 percent of the Celect IVC Filters manufactured by Cook Medical, Inc. puncture the wall of the inferior vena cava within two months.
Recent studies have concluded that over 40 percent of the Celect IVC Filters manufactured by Cook Medical, Inc. puncture the wall of the inferior vena cava within two months.
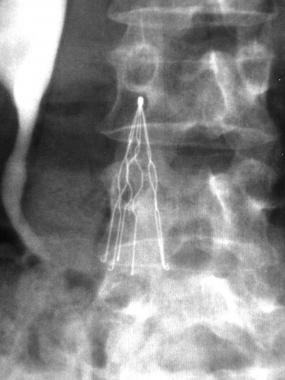
Inferior vena cava (IVC) filters like the Celect devices are shaped like a cone, with up to 12 needle-like wire legs organized around a central hook. The purpose of these legs is to attach into the wall of the inferior vena to firmly anchor it inside the vein. However, the walls of the vena cava are extremely thin and easily punctured.
To make matters worse, vena cava is continually pulsating due to blood pressure, breathing, and other movements, and IVC filters must be built to withstand constant flexing without breaking. If they are too rigid, the legs will puncture the wall of the vein and cause serious health issues for the patient.
IVC Filter Lawsuits
Approximately 2,350 IVC filter lawsuits are pending in multidistrict litigation (MDL) in the U.S. District Court for the Southern District of Indiana (Indianapolis Division). The cases make similar allegations against Cook Medical about the serious complications associated with the company’s IVC filters, including:
- Filter fracture, in which the IVC filter legs (struts) break off and threaten cardiac tissue and heart function.
- Tilt, which can decrease the filter’s ability to trap clots and make the IVC filter more difficult to remove.
- Migration, which occurs when the filter detaches from the vena cava’s walls where it perforate vessel walls, tissues, and the heart, blocking blood flow.
- Perforation of the vena cava, which can lead to retroperitoneal hematoma, gastrointestinal bleeding, and sepsis.
The lawsuits also claim that Cook Medical, Inc. marketed and sold a defective medical device, misrepresented the product in marketing claims, and failed to warn medical professionals and patients of the risks associated with their IVC filters.
Other IVC Filter Lawsuits News
An Arizona judge denied class certification for Bard IVC filter lawsuits, citing differences among plaintiffs' devices and claims. These lawsuits allege serious injuries from defective IVC filters and insufficient risk warnings.
A new study suggests IVC filters may increase pulmonary embolism risks in traumatic spine injury patients, raising concerns about their safety compared to other preventive treatments.
A recent study suggests IVC filters may be overused in managed care, often left in place longer than FDA guidelines recommend, raising concerns about patient safety and medical oversight.
Many IVC filters remain in patients indefinitely despite FDA recommendations for removal, raising concerns about serious complications such as fracture, migration, and vena cava perforation.
A new bellwether schedule has been issued in Bard IVC filter litigation, as thousands of lawsuits move forward alleging serious complications linked to the company’s retrievable devices.
Eight plaintiffs allege Cordis IVC filters caused serious complications, citing studies linking the devices to high fracture rates and claiming the company downplayed known risks in lawsuits now pending.