Research findings released by Humana Inc. suggest that inferior vena cava (IVC) filters may be overused among managed care populations and not always removed when they are no longer necessary.
The study, published in the Journal of Thrombosis and Thrombolysis, compared the results of managed care patients who received IVC filters and patients who did not have them, although they may have been considered viable candidates. The researchers concluded that patients who received filters were more frequently hospitalized (and re-hospitalized) than those without IVC filters
Although IVC filters are typically only recommended for patients who cannot use blood thinners, the study found that anticoagulant use was greater in patients who had not received filters, implying that IVC filters are frequently used in cases what do not meet clinical practice guidelines.
IVC Filters Often Left in Place, Despite FDA Recommendations
The Humana researchers also found IVC filters were actually removed in only a small percentage of patients — six percent among patients with a history of deep vein thrombosis (DVT) or pulmonary embolism (PE), and 16 percent of those whose IVC filters were there to prevent DVT or PE following surgery. In May 2014, the FDA recommended that doctors remove IVC filters within a month or two after the risk of pulmonary embolism had passed, and not leave them in place indefinitely, noting that many doctors were not adequately warned about the importance of retrieving the devices.
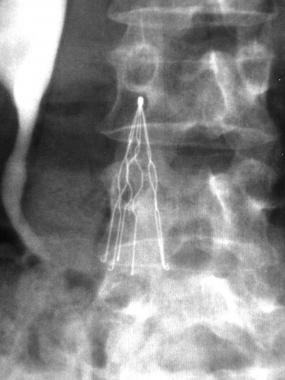
The IVC (inferior vena cava) filter is a spider-like medical device used to filter blood clots and prevent them from reaching a patient’s lungs and causing a pulmonary embolism, which can result in permanent lung damage or even death. Retrievable IVC filters are intended to be removed once the threat of pulmonary embolism has passed. There are currently more than 3,000 IVC filter lawsuits against Cordis Corporation, C.R. Bard, Cook Medical, B. Braun, Rex Medical, and others pending in state and federal courts around the nation.
Other IVC Filter Lawsuits News
An Arizona judge denied class certification for Bard IVC filter lawsuits, citing differences among plaintiffs' devices and claims. These lawsuits allege serious injuries from defective IVC filters and insufficient risk warnings.
A new study suggests IVC filters may increase pulmonary embolism risks in traumatic spine injury patients, raising concerns about their safety compared to other preventive treatments.
Studies reveal that Cook Medical’s Celect IVC filters may puncture the vena cava within two months, leading to serious complications and fueling thousands of lawsuits alleging defective design and failure to warn.
Many IVC filters remain in patients indefinitely despite FDA recommendations for removal, raising concerns about serious complications such as fracture, migration, and vena cava perforation.
A new bellwether schedule has been issued in Bard IVC filter litigation, as thousands of lawsuits move forward alleging serious complications linked to the company’s retrievable devices.
Eight plaintiffs allege Cordis IVC filters caused serious complications, citing studies linking the devices to high fracture rates and claiming the company downplayed known risks in lawsuits now pending.