Bard IVC Filter Lawsuits
Over 2,000 IVC filter lawsuits are currently pending in the District of Arizona. The cases make similar allegations against Bard, including:
- Bard IVC filters are associated with high rates of fracture, migration, and tilting.
- Bard IVC filters have been associated with perforation of the inferior vena cava, internal bleeding, respiratory distress, embolization, and other life threatening complications.
- R. Bard failed to provide doctors with adequate warnings regarding the importance of filter removal, since failure to retrieve an IVC filter in a timely manner has been shown to greatly increase the risk of injury and complications, according to the U.S. Food and Drug Administration (FDA).
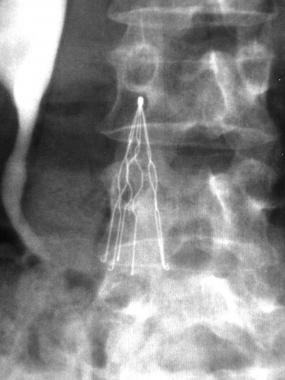
What are IVC Filters?
The IVC (inferior vena cava) filter is a spider-like medical device used to filter blood clots and prevent them from reaching a patient’s lungs and causing a pulmonary embolism, which could mean permanent lung damage or even death. Retrievable IVC filters, such as C.R. Bard’s Recovery and G2 IVC filters, are intended to be removed once the threat of pulmonary embolism has passed.
Since 2010, the FDA has issued two safety alerts connected with the use of retrievable IVC filters. The first was in August 2010, after the devices were the subject of hundreds of adverse event reports, including fracturing and migrating to other parts of the body, where they perforated organs and blood vessels. In May 2014, a second alert was issued to update the 2010 communication and remind doctors of the importance of retrieving the filters from the body once the danger of pulmonary embolism has passed.