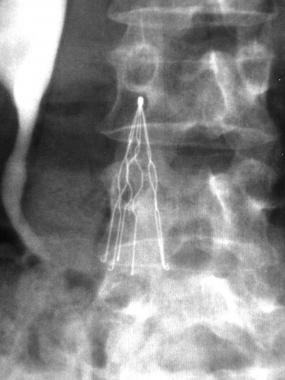
A Texas man who had an Option ELITE Retrievable Inferior Vena Cava (IVC) filter surgically implanted in 2015 to prevent a pulmonary embolism (PE) and was diagnosed with diagnosed with blood clots in both lungs four months later has filed a lawsuit for damages in the Philadelphia Court of Common Pleas.
The plaintiff’s doctors also discovered blood clots lodged in his IVC filter and his left iliac vein, requiring emergency surgery. Later that same day, the man underwent venogram and thrombolysis to locate and dissolve the blood clots. Both procedures were unsuccessful and the clots were not removed, so a day later, his doctors performed an AngioJet Thrombectomy to attempt to remove the clots, which was also unsuccessful. Because of the presence of these clots, the patient’s IVC filter is permanent and cannot be removed.
As with other IVC filter cases, this lawsuit accuses IVC manufacturer Rex Medical of selling a defective and unreasonably dangerous medical device and failing to conduct sufficient clinical testing to determine how the filter would function once implanted permanently in the body of a patient. There are now more than 3,500 IVC lawsuits pending in state and federal courts nationwide against various medical device manufacturers, including Rex Medical, C.R. Bard, Cook Medical, and B. Braun.
FDA: IVC Filters Should be Removed as Soon as Possible
In May 2014, the U.S. Food and Drug Administration (FDA) updated an August 2010 warning concerning adverse event reports with IVC filters. In the update, the FDA encouraged all physicians involved in the treatment and follow-up of patients receiving IVC filters to consider the risks and benefits of filter removal for each patient. The update also required manufacturers to collect additional clinical data addressing safety questions for currently marketed IVC filters in the U.S.
Other IVC Filter Lawsuits News
An Arizona judge denied class certification for Bard IVC filter lawsuits, citing differences among plaintiffs' devices and claims. These lawsuits allege serious injuries from defective IVC filters and insufficient risk warnings.
A new study suggests IVC filters may increase pulmonary embolism risks in traumatic spine injury patients, raising concerns about their safety compared to other preventive treatments.
Studies reveal that Cook Medical’s Celect IVC filters may puncture the vena cava within two months, leading to serious complications and fueling thousands of lawsuits alleging defective design and failure to warn.
A recent study suggests IVC filters may be overused in managed care, often left in place longer than FDA guidelines recommend, raising concerns about patient safety and medical oversight.
Many IVC filters remain in patients indefinitely despite FDA recommendations for removal, raising concerns about serious complications such as fracture, migration, and vena cava perforation.
A new bellwether schedule has been issued in Bard IVC filter litigation, as thousands of lawsuits move forward alleging serious complications linked to the company’s retrievable devices.