One of the first recipients of a Bard Eclipse Vena Cava filter in 2010 has filed a lawsuit alleging filter fracture against device maker C.R. Bard. The lawsuit, filed by an Illinois woman, will be centralized with over 1,360 others now pending against Bard in multidistrict litigation (MDL) in Arizona.
One of the first recipients of a Bard Eclipse Vena Cava filter in 2010 has filed a lawsuit alleging filter fracture against device maker C.R. Bard. The lawsuit, filed by an Illinois woman, will be centralized with over 1,360 others now pending against Bard in multidistrict litigation (MDL) in Arizona.
The cases pending in the MDL have common allegations, among them that C.R. Bard sold the defective medical devices when the company knew or should have known that the IVC filters posed unreasonably dangerous safety risks, and failed to warn patients about the dangers.
What are IVC Filters?
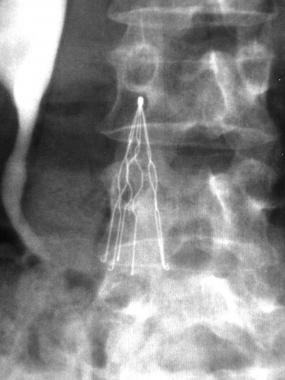
The IVC (inferior vena cava) filter is a spider-like medical device used to filter blood clots and prevent them from reaching a patient’s lungs and causing a pulmonary embolism, which can result in permanent lung damage or even death. Retrievable IVC filters, such as C.R. Bard’s Recovery and G2 IVC filters, were intended to be removed once the threat of pulmonary embolism has passed.
Design Flaw?
Despite IVC design changes made by Bard, continuing fractures suggested an ongoing design flaw with early generation Bard filters that was not remedied until June 2013 with the 6th generation Denali, an IVC filter manufactured with completely new materials and single-piece laser-cutting technology.
The Denali is the only Bard retrievable filter still on the market, and all previous generations – Recovery, G2, G2 X, Eclipse, and Meridian – were only sold for a few years before being taken off the market due to high rates of fracture, migration, and embolization of broken fragments in the bloodstream. Twenty-seven deaths and over 300 injuries have been associated with the Recovery, a 1st generation Bard retrievable filter on which all future generations were based, according to a 2015 NBC News report.
Other IVC Filter Lawsuits News
An Arizona judge denied class certification for Bard IVC filter lawsuits, citing differences among plaintiffs' devices and claims. These lawsuits allege serious injuries from defective IVC filters and insufficient risk warnings.
A new study suggests IVC filters may increase pulmonary embolism risks in traumatic spine injury patients, raising concerns about their safety compared to other preventive treatments.
Studies reveal that Cook Medical’s Celect IVC filters may puncture the vena cava within two months, leading to serious complications and fueling thousands of lawsuits alleging defective design and failure to warn.
A recent study suggests IVC filters may be overused in managed care, often left in place longer than FDA guidelines recommend, raising concerns about patient safety and medical oversight.
Many IVC filters remain in patients indefinitely despite FDA recommendations for removal, raising concerns about serious complications such as fracture, migration, and vena cava perforation.
A new bellwether schedule has been issued in Bard IVC filter litigation, as thousands of lawsuits move forward alleging serious complications linked to the company’s retrievable devices.