C.R. Bard is one of several manufacturers dealing with allegations of defective inferior vena cava (IVC) filters, and the company’s 10-Q report filed with the U.S. Securities and Exchange Commission on September 30 reveals that 1,055 lawsuits are pending against Bard in various federal and state jurisdictions.
The Bard multidistrict litigation (MDL) was formed in August 2015 when more than 1,000 lawsuits were consolidated into MDL 2641 in the District of Arizona. Another 50 cases are currently pending in various state courts, and approximately 40 more have been threatened but have not yet been filed. Bard IVC filter lawsuits are also pending in Canada, where three class action IVC filter lawsuits were filed in three separate provinces in 2016: Quebec, Ontario, and British Columbia.
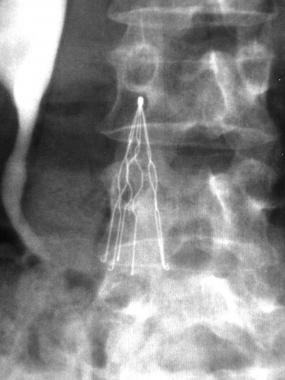
Issues with Bard’s IVC Filters
IVC filters like C.R. Bard’s Recovery and G2 are designed to catch blood clots and prevent them from making their way to the lungs where they could potentially cause a pulmonary embolism. But problems with the filters have prompted hundreds of adverse reports to the U.S. Food and Drug Administration (FDA).
• In August 2010, the FDA issued a warning about the risks surrounding IVC filters, particularly when the devices broke apart and traveled to other areas of the body and caused perforations and other serious injuries.
• In May 2014, the FDA updated this earlier communication, recommending that doctors remove IVC filters within a month or two after the risk of pulmonary embolism had passed, and not leave them in place indefinitely, noting that many doctors were not adequately warned about the importance of retrieving the devices.
Most IVC filters are never removed because insufficient clinical follow-up, failed retrieval procedures, or because patients are not offered the opportunity for filter removal, according to the FDA.