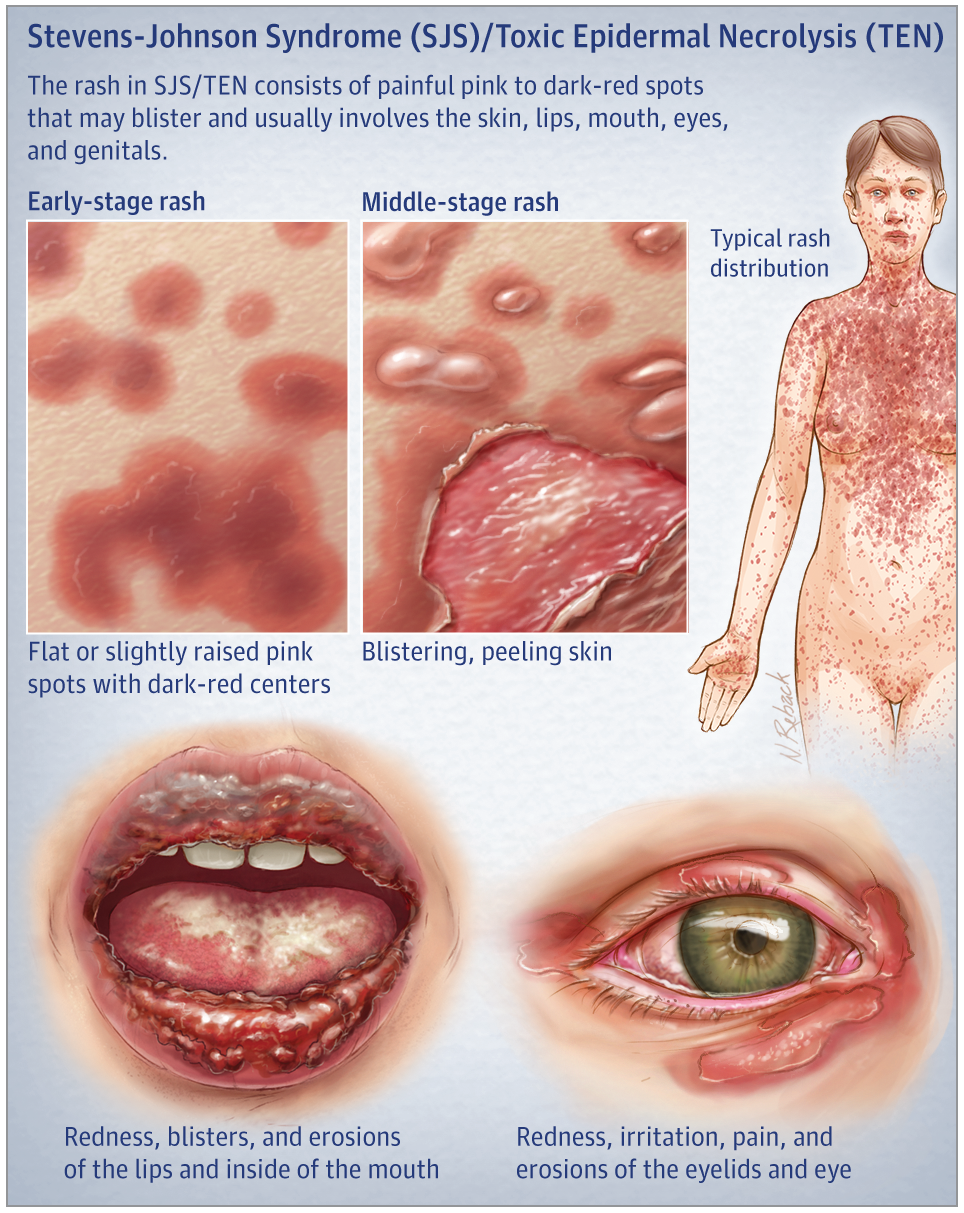
Stevens-Johnson Syndrome (“SJS”) is an extremely painful and potentially lethal condition that can be caused by several medications, including Lamictal.
Childers, Schlueter & Smith has successfully resolved numerous lawsuits involving Lamictal, SJS, and the negligence of healthcare providers involved in both the prescribing of this medication and the diagnosis, or rather missed diagnosis, of SJS, and is currently litigating several such cases around the country.
What is Lamictal?
Lamictal, also known as lamotrigine, is approved by the FDA for treating patients diagnosed with epilepsy and Bipolar Disorder. We have seen many instances, however, of psychiatrists or other medical providers improperly prescribing Lamictal “off label” to treat other psychiatric issues such as generalized depression. Importantly, Lamictal has not been proven to be effective for the treatment of manic episodes in Bipolar patients. While Lamictal is approved for use in pediatric patients suffering from epilepsy, it is not approved for use in pediatric patients with Bipolar Disorder or any other mood disorders.
Regardless of its intended use, any doctor prescribing Lamictal, and any pharmacist dispensing Lamictal, should be aware of the association between Lamictal and SJS, and should provide their patients with proper counseling about these risks.
The FDA has required that the manufacturers of Lamictal include a black box warning on its package specifically related to SJS. Lamictal is the only mood stabilizer on the market in the United States with a Black Box Warning for Serious Skin Reactions, including SJS. A Black Box Warning, mandated for the safety of patients prescribed Lamictal, is the most serious of the five (5) levels of warnings contained on a drug’s label. The black box warning for Lamictal states:

- Lamictal use has been linked to cases of life-threatening rashes, including Stevens-Johnson Syndrome.
- Additional Factors that may increase the risk of rash include:
- co-administration with valproate (Depakote),
- exceeding the recommended initial dose of Lamictal, or
- exceeding the recommended dose escalation for the drug.
- Lamictal can also cause benign rashes, but because it is not possible to predict which rashes might be serious or life-threatening, Lamictal should be discontinued at the first sign of rash, unless the rash is clearly not drug-related.
- The rate of serious rash is greater in pediatric patients than in adults.
Lamictal Use Potentially Linked to Stevens-Johnson Syndrome – (READ MORE)
In order to minimize the risk of Serious Skin Reactions, the FDA approved dosing instructions for Lamictal require that a patient be started at a low dose (typically 25 mg per day) for at least 2 weeks, followed by an initial increase of dose (typically 50 mg per day) for at least 2 weeks, before the effective dose (typically 100 mg per day) can be started after at least 4 weeks of being on the lower doses. The FDA’s approval of the dose escalation regimen is intended to protect the safety of patients prescribed Lamictal. Despite clear warnings from the FDA, many healthcare professionals continue to prescribe Lamictal to patients who may not be appropriate candidates for the medication, initiate the medication at too high of a starting dose, escalate the doses of the medication too quickly, and/or fail to provide any guidance to their patients about this potentially deadly side effect.

Prompt discontinuation of Lamictal and treatment of SJS, particularly in a specialty unit or burn unit, can greatly reduce the symptoms of the condition and may even save the patient’s life. This is why proper guidance and dosing is critically important to patient safety, and why it is important for patients who are taking Lamictal to carefully watch for any signs of a rash and/or irritation of their eyes, mouth, and/or genitalia, and to both seek immediate treatment and contact their prescribing physician or pharmacist if any such symptoms appear.
If you or a loved one has developed SJS or its more severe form, Toxic Epidermal Necrolysis (TEN), please contact us immediately to speak with one of our attorneys who specialize in these cases. Many law firms claim to handle SJS cases, but very few actually do. Selecting the right firm is crucial to the success of your case. Childers, Schlueter & Smith has successfully resolved numerous lawsuits involving Lamictal, SJS. We may be able to help. Contact us to share your story via our website or call.