Despite a recommendation by the U.S. Food and Drug Administration (FDA) that retrievable inferior vena cava (IVC) filters be removed when they are no longer needed, they are often left in place indefinitely.
Despite a recommendation by the U.S. Food and Drug Administration (FDA) that retrievable inferior vena cava (IVC) filters be removed when they are no longer needed, they are often left in place indefinitely.
There are currently no guidelines on the time frame during which IVC filters must be retrieved from the body, and according to the U.S. Food and Drug Administration (FDA), the majority of IVC filters are not retrieved, despite the perceived risk of increased fracture, embolism, and IVC wall penetration.
What are IVC Filters?
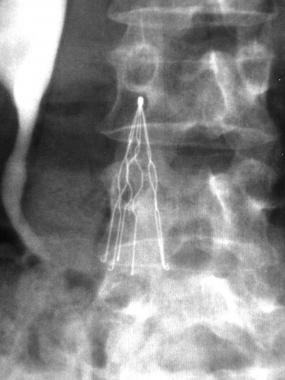
IVC filters are medical devices placed between the proximal vessels of the lower extremities and the right side of the heart, where they help prevent pulmonary embolism by catching blood clots and preventing them from traveling to the lungs.
Over the past 30 years, the use of the IVC filter has steadily increased. While approximately 2,000 filters were implanted in patients in the U.S. in 1979, by 1990, over 120,000 IVC filters had been placed. At the end of the decade, nearly 50,000 IVC filters were being implanted each year.
FDA Adverse Event Reports
Since 2005, the U.S. Food and Drug Administration (FDA) has received more than 900 reports of adverse events connected to IVC filters. According to the FDA, the majority of IVC filters are not retrieved despite the risk of increased fracture, embolism, and IVC wall penetration.
Common complications associated with IVC filters left in the body for long periods of time include:
- The progression of DVT
- Recurrent PE
- Filter migration
- Tilt, break, or embolism
- IVC perforation or occlusion
A 2013 JAMA Internal Medicine report noted that an attempt is made to remove only about 10.5 percent of all IVC filters implanted, and concluded that this low retrieval rate, combined with other factors, resulted in less-than-optimal outcomes due to high rates of venous thromboembolism (VTE).