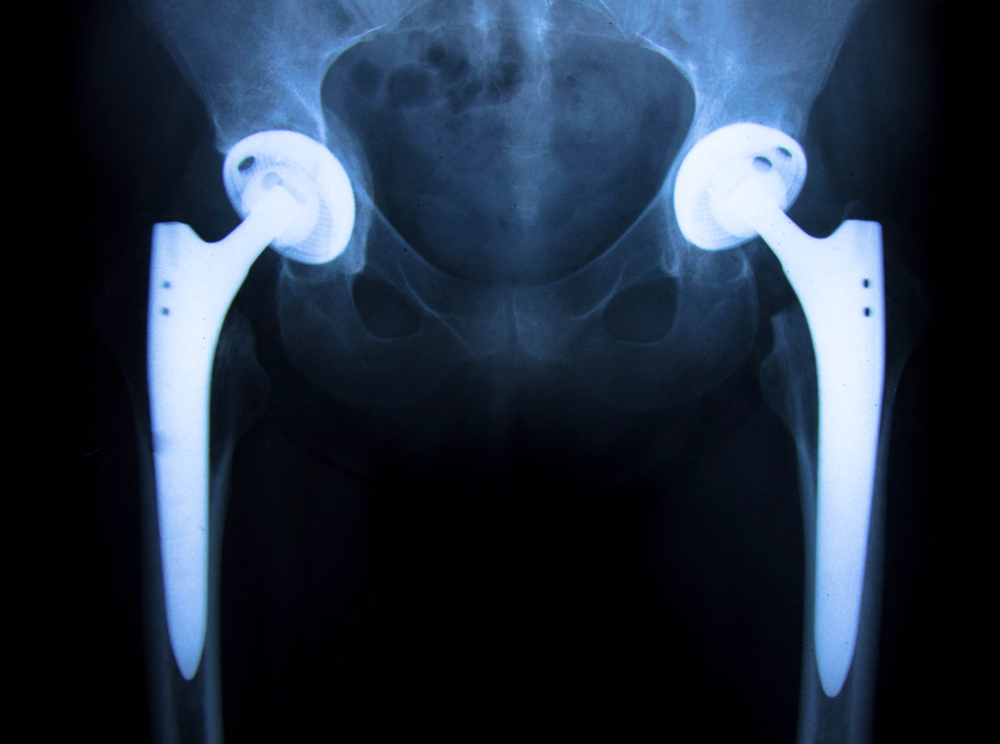
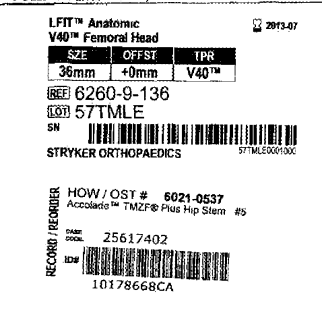
Stryker has faced numerous hip replacement recalls and lawsuits in the past, and it seems likely that the company may be looking at more of the same related to its LFIT Anatomic CoCr V40 femoral heads. The V40 heads were used in the following Stryker products:
Stryker has faced numerous hip replacement recalls and lawsuits in the past, and it seems likely that the company may be looking at more of the same related to its LFIT Anatomic CoCr V40 femoral heads. The V40 heads were used in the following Stryker products:
- Accolade TMZF
- Hfx
- Citation
- LASST
- Accolade II
- Anato
- Secur-Fit Advanced
- LSP76
These hip replacement products are modular components used in total hip replacement procedures, and when defective, can lead not only to increased failure rates for the hip replacements but also serious side effects. The problem with the components is related to the taper locks that connect the femoral head to the femoral neck, which appear to have a relatively high rate of failure and exhibit the following symptoms:
- Metal in the blood or other tissues
- Negative tissue reaction to the components
- Inflammation
- Instability in the joints
- Fractured bones
- Dislocation
- Loss of mobility
- Pain
- Noise related to the implant
These symptoms can cause significant pain and severe problems for patients, who are urged to contact their physicians immediately.
Where there is micromotion between the stem and the head, the metal becomes scratched from the movement and corrosion takes place. This corrosion allows metal particles to leach into the hip compartment, causing a condition called metallosis where the positively charged metal ions damage the tissue in the hip compartment. The tissue damage progresses to necrosis or death of the tissue and causes both pain and inflammation.
In August, Stryker sent an alert to medical professionals, advising them that the LFIT V40 femoral heads, manufactured between 2001 and 2011, had been involved in a higher than expected series of product failures. None of the products were removed from the shelves because they had all been either implanted in patients or were expired.