CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
Stryker Hip Implant News Update October 2018

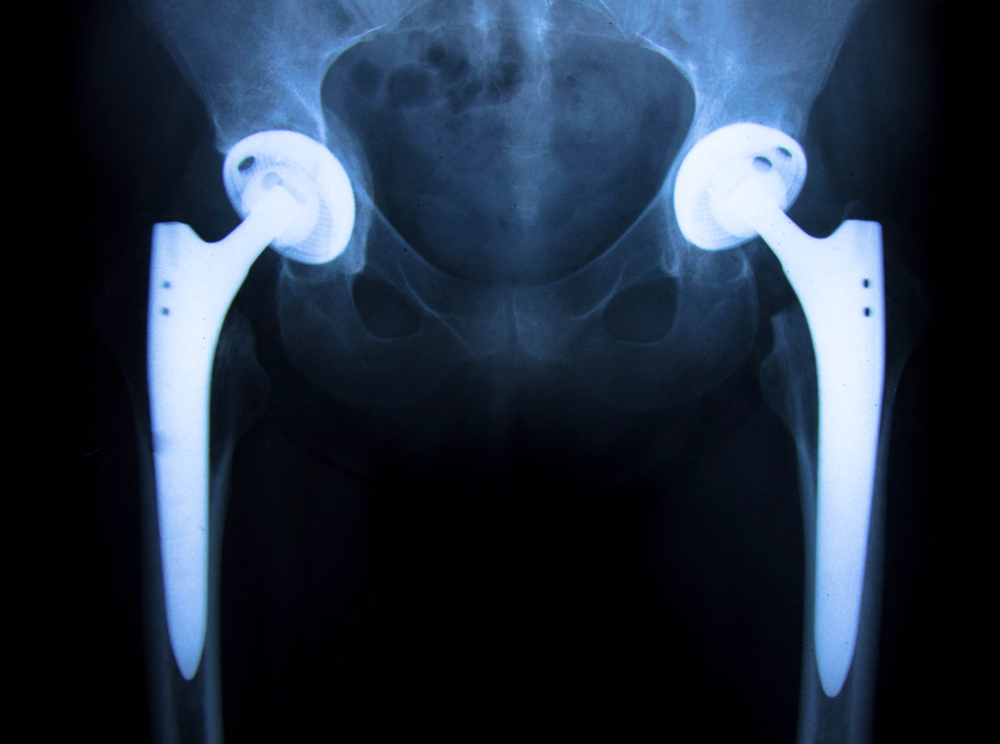
Have you had hip replacement prior to 2012? Medical device manufacturer, Stryker, has acknowledged V40 heads in their hip replacements manufactured prior to March 4, 2011 have an increased failure rate for head/hip stem dissociation. Stryker V40 heads were distributed to health care professionals relatively close the manufacturing date.
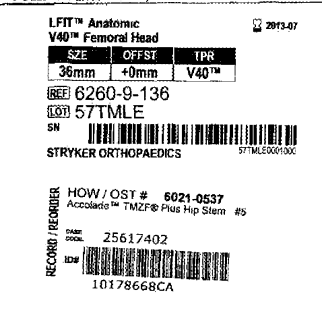
In 2016, Stryker issued an extended recall on metal head, hip implants to include anatomic LFIT V40 heads with catalog numbers beginning “6260 -9”. More specifically, product safety notifications sent to health care professionals May 22, 2018 was an extension of the prior recall that primarily affected the size 40 and size 44mm V40 heads. The specific catalog numbers, head diameters, and offset data of effected devices are contained in the following chart:

“We believe the defective nature of this component is not confined to the expansion of this list and others are still at risk”
Full notification: Expanded Product Safety Notification
In review of records and other information, the V40 made of Cobalt and Chrome (CO/CR) are worth investigating for a potential claim. This is especially true if the stem is on the earlier version Accolade TMZF stem. We do not think hip implants with ceramic heads will be viable claims.
Failures with cobalt/chrome heads have also been seen in cases filed in the MDL. While these failures are less common with Accolade 2, Restoration, Citation, Meridian and Securfit with V40 adapter, Childers, Schlueter and Smith continue to represent and file cases for claimants that have cobalt/chrome heads that do not match the recall or health hazard notifications issued thus far. We believe the defective nature of this component is not confined to the expansion of this list and others are still at risk.
What are the latest updates on the litigation in Federal Court?
Since the last post, over 300 cases have been filed and consolidated in MDL 2768 in United States District Court of Massachusetts before Judge Talwani. Richard Schlueter of Childers, Schlueter, and Smith LLC was selected by appointment to serve on the Plaintiff’s Steering committee by court order on June 30th 2017. Bellwether trials are currently being scheduled with the first trials slated to begin in the first quarter of 2020. The Defense for Stryker and Plaintiff’s counsel have each selected a total of 9 cases each for the initial Bellwether pool.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.
Stryker’s track record includes multiple recalls and lawsuits involving defective hip implants—and recent reports suggest continued failures in other device components, raising serious safety concerns.