CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
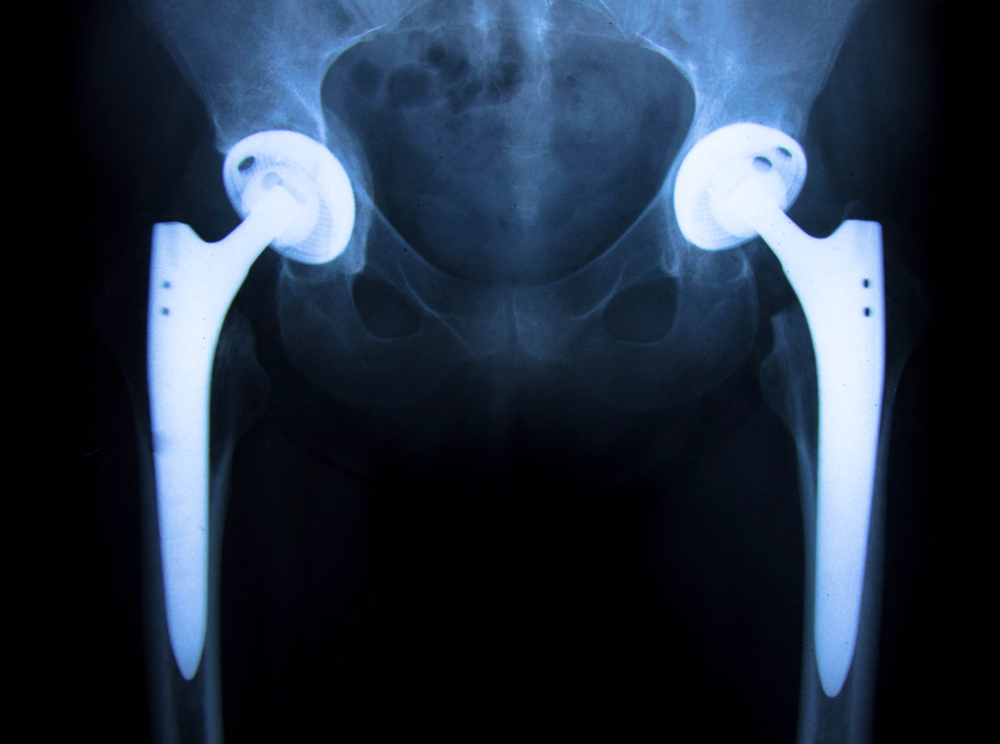
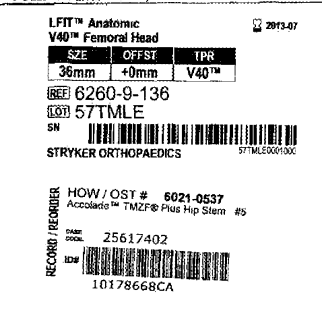
These images are the chart sticks of a recent client of ours that had a catastrophic failure of a Stryker Accolade 40 LFit Cobalt / Chrome combination. Our firm , Childers Schlueter and Smith, LLC filed 5 cases in Federal Court last month concerning revisions of the V40 combination either because of disassociation (coming apart), or from taper corrosion, (between the head and the stem) last month. This appears to be a very important health hazard that the consumer is mostly unaware that are believed to affect tens of thousands of patients that in our humble opinion should be monitored. The cases filed all are trunnion failures of the titanium stem that are matched with the optional versions of V40 heads made of Cobalt and Chromium rather than the optional ceramic equivalent. It is our belief in review of emerging literature and experience in past recalls that these filings are the tip of the iceberg and a wave of failures will be discovered over time.
The current cases are being consolidated and sent to the Multi District litigation Docket ( MDL )from around the country before Judge Talwani in MDL 2768 that was recently created by the Judicial Panel of Multi District litigation. Anyone interested can follow this link through the Federal Pacer System and see the current status of filings on the Master Federal Docket.
Currently there are 72 cases consolidated before the court as of June 1, 2017. More cases are being filed and added to the docket weekly. Everyone is asking, how big is the problem with the Stryker Accolade/V40 combination? How many are expected to fail? Time will tell for sure but the big issue is patient education and notification. Most patients are not aware of the type of prosthetic that they have implanted. Many patients may know from memory that they have a Stryker implant or have a Stryker card in their wallet. Unfortunately, having the card or knowledge of Stryker as the manufacturer does little in identifying the actual components used. Stryker has many great products in use today and in our experience most patients with other models and combinations are not at risk and don’t suffer from the failures known to exist in the Accolade V40 Cobalt/Chrome combination. Its like knowing you have a Chevrolet or a Ford –it’s the model that is important like a Prius or Mustang not knowing only the maker. Stryker has not issued guidelines or bulletins that we are aware in encouraging testing and follow up for patients. Most surgeons are in the dark of what to do and are following up with patients that present in their office with problems that voluntarily follow up unaware of the risk of failure. Further, unlike the recall the manufacturer had with its Rejuvenate and ABG2 model in paying for testing and out of pocket expenses of patients, Stryker has not instituted a program to compensate and pay for testing. Unfortunately, this will have the effect of delayed diagnosis and, I predict a greater unexpected incident rate of catastrophic failures of incidents. Stay tuned and informed if you are at risk with one of these products.
Given our work on previous Stryker Hip Implant Recalls, we know a lot about Stryker and their products. For this reason and others, Childers Schlueter & Smith continues to investigate and pursue Stryker LFIT V40 Femoral head claims associated with the Accolade TMZF stem. If you have questions about your hip implant system, please call our Stryker Hip Implant Lawyers or send us a confidential message at cssfirmllcdev.wpenginepowered.com or email us at intake@cssfirmllcdev.wpenginepowered.com.