CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
About Stryker Hip Implant Lawsuits
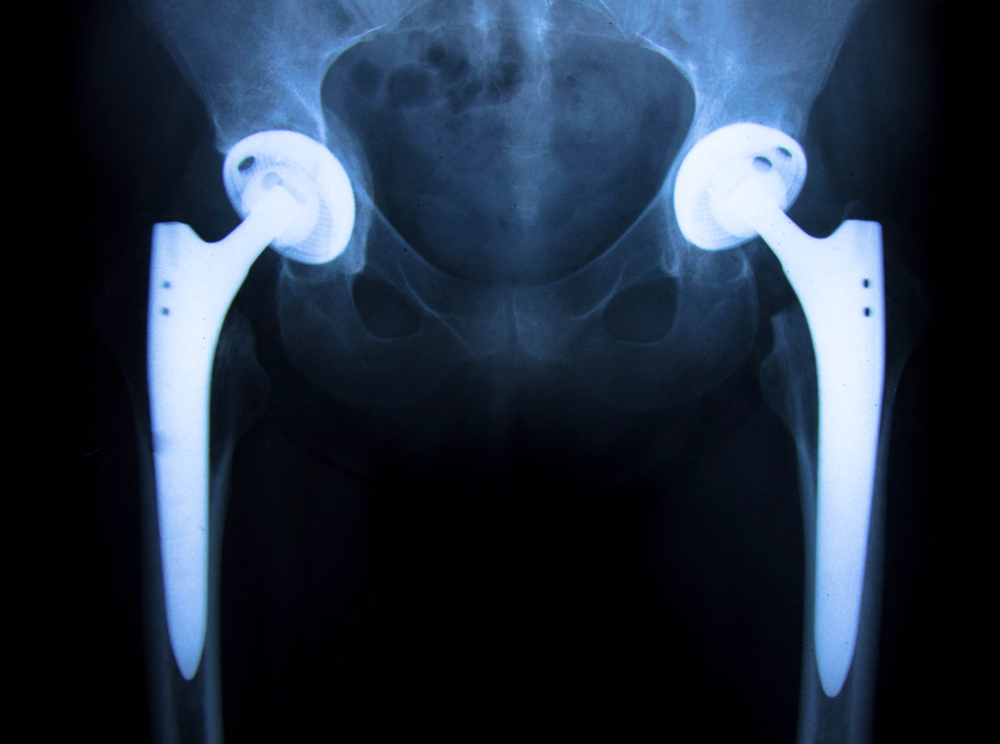
- The LFit Anatomic V40 femoral head has been recalled due to taper lock failures, which can result in serious complications such as joint disassociation, metallic debris, and fractured hip stem trunnions. This recall joins earlier recalls of Stryker’s Rejuvenate and ABG II hip implants, both of which have faced lawsuits for high failure rates and metal corrosion.
- Patients with recalled Stryker hip implants have reported serious side effects, including joint pain, metallosis (metal poisoning), osteolysis (bone loss), and tissue necrosis. Many have required revision surgeries to address these issues.
- Stryker has spent over $1 billion to resolve lawsuits resulting from these recalls. Victims of defective implants are seeking compensation for physical, emotional, and financial hardships caused by these defective devices.
Why Hire CSS for a Stryker Hip Implant Lawsuit
Childers, Schlueter & Smith has a proven track record in handling complex hip implant litigation, including Stryker cases. With extensive experience in product liability and medical device lawsuits, our attorneys have helped numerous clients nationwide secure compensation for their medical expenses, pain, and suffering. We understand the technical details of these cases and will work tirelessly to ensure justice is served.
By choosing CSS, you gain a team of dedicated, knowledgeable attorneys who will guide you through every step of the process, fight for your rights, and ensure that you receive the compensation you deserve. Contact us today for a free case evaluation. Time is limited, so don’t delay!
Stryker Hip Implant Complications
Stryker may announce a new hip implant recall concerning select LFit V40 femoral heads in the very near future due to higher-than-expected complaints of taper lock failures. These soon-to-be-recalled Stryker hip implant femoral heads are known to be utilized on both Accolade TMZF and Accolade 2 stems as well as Meridian and Citation stems. Dangers from these failures include: disassociation of the femoral head from the hip stem, fractured hip stem trunnion, excessive metallic debris, and numerous other complications.
In addition to the LFit V40 failures, the medical device company Stryker is facing thousands of lawsuits from people who have suffered complications from the company’s Rejuvenate and ABG II hip implants. If you or a loved one has been harmed by one of these recalled hip implants, you should join these people to seek the compensation you deserve to ensure the best recovery from this painful ordeal.
The dedicated product liability attorneys at Childers, Schlueter & Smith have been at the forefront of this nationwide litigation, helping negotiate settlements on behalf of injured Americans. We know defective hip lawsuit cases inside and out. That’s why you should give us a call today if you or a loved one has suffered due to a defective ABG II or Rejuvenate hip replacement.
Time is extremely limited to participate in this litigation, so don’t delay in speaking with our Stryker hip implant lawyers.
Stryker Rejuvenate and ABGII Hip Implant Global Settlement Announced on Nov. 3, 2014.
The pain associated with a problematic hip replacement can be agonizing. Reports on file with the Food and Drug Administration tell of patients unable to walk and suffering other complications.
Complications associated with Stryker’s recalled ABG II and Rejuvenate hip systems include:
- Joint pain, which often leads to difficulty walking.
- Inflammation
- Metallosis, or metal poisoning. This occurs when components of the implant grind together, sending metal shards into the body.
- Osteolysis, or bone dissolution. This can lead to broken bones or implant failure.
- Joint failure or loosening. If the implant fails, your hip cannot function as it should. This requires revision surgery.
- Necrosis, or tissue death. This may occur in the area surrounding the hip implant.
Which Models Were Recalled?
Stryker recalled its Rejuvenate and ABG II modular-neck hip stems in June 2012. The recall came after the devices were linked to high failure rates and corrosion of the joint’s metal components, which can damage surrounding tissue. Many people with one of these two types of hip implants have had to undergo revision surgery to fix these problems. Anyone experiencing issues with their Stryker hip implant should reach out to a lawyer right away.
Why Are People Suing Over Recalled Stryker Hips?
None of the plaintiffs in Stryker hip implant lawsuits wanted to end up in this situation. All you and other hip victims wanted was a hip replacement that did its job.
The complications these recalled Stryker hips have caused are more than just painful. They also place a huge physical, emotional, and financial burden on patients and their families. Addressing these hip complications often requires subjecting the patient to a revision surgery – an expensive procedure with a lengthy recovery period.
If you are the victim of a defective hip, you don’t deserve this.
Nothing will take back the pain that this ordeal caused. However, taking legal action may be able to get you the resources you need to ensure that the suffering stops right here. The future can be brighter.
In October 2013, the Wall Street Journal said that Stryker estimated spending as much as $1.13 billion to resolve the lawsuits that stem from the recall of these two products.
What Is a Metal-on-Metal Hip Implant?
Metal-on-metal hip implants were originally marketed as an alternative to orthopedic implants coated with plastic or ceramic. Based on laboratory testing, the all-metal implants were shown to be more resistant to wear and to reduce the risk of dislocation. For a decade, surgeons favored metal-on-metal implants.
Yet it now appears that the devices are prone to early failure. Medical researchers have voiced concern over the years about the safety of metal-on-metal hip devices due to the effects of metal components rubbing against each other. That’s why some hip systems by Stryker and other manufacturers have been recalled.
Patients who are at higher risk include, but are not limited to:
- Female patients
- Patients with suppressed immune systems
- Patients who are severely overweight
- Patients with high levels of physical activity
Stryker released its Rejuvenate and ABG II in 2009 but recalled the devices three years later in July 2012. Stryker’s metal-on-metal hip implant began to show evidence of corrosion after the devices were implanted.
It has been alleged that the ABG II and Rejuvenate hip systems were not sufficiently scrutinized before being approved for the market. That’s because they were approved under a provision known as 501(k), which allowed the hip implants to be approved without a vigorous demonstration of their safety because they are similar in design to another product already approved.
Critics are calling on the FDA to close this “loophole.”
Our Experienced Stryker Hip Implant Attorneys Want to Help
If you or a loved one received a Stryker hip implant and have experienced complications or have had to undergo revision surgery as a result, contact our Stryker hip implant lawyers today for a free case evaluation to learn about your legal options.
There is no obligation. Our attorneys will work closely with you to seek justice and fight for compensation for your injuries.
CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
Frequently Asked Questions
Common complications include joint pain, metallosis (metal poisoning), osteolysis (bone dissolution), implant loosening, tissue death (necrosis), and the need for revision surgery.
Metal-on-metal implants were initially favored for their durability, but they have been linked to early failure and complications due to metal components grinding against each other, leading to metallosis.
Yes, if you or a loved one has experienced complications or required revision surgery due to a defective Stryker hip implant, you may be eligible to file a lawsuit to seek compensation.
Higher-risk groups include women, individuals with suppressed immune systems, those who are severely overweight, and patients with high levels of physical activity.
Compensation may cover medical expenses, pain and suffering, lost wages, and the cost of revision surgery.
The ABG II and Rejuvenate hip systems were approved under a 501(k) provision, which allowed them to be fast-tracked due to their similarity to other approved devices. Critics have called for this loophole to be closed.