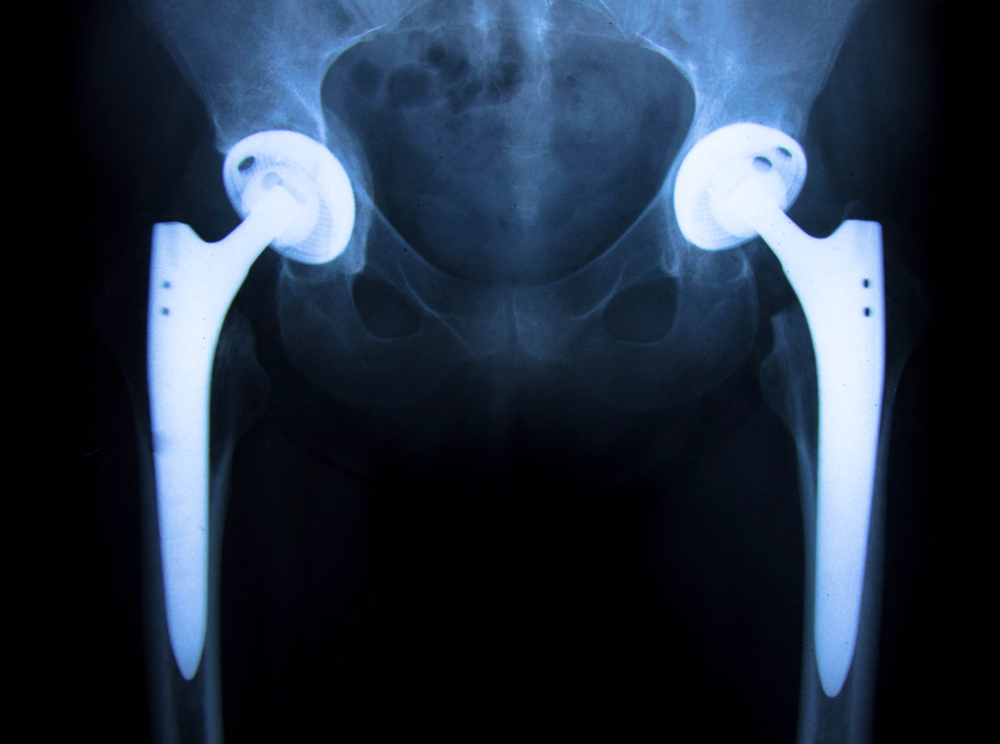
Many problems have been associated with the faulty Stryker hip implant recall. According the FDA, they have received numerous reports from patients about serious side affects associated with the Stryker Rejuvenate and ABG II modular-neck stems hip implants.
On July 6, 2012, Stryker voluntarily recalled its Rejuvenate and ABG II modular-neck stems. The problems patients were having with the faulty hip implants include:
- Necrosis
- Metallosis
- Osteolysis
- Loosening of implant
- Hypothyroidism
- Damage to bone and tissue surrounding the hip joint
- Infection
- Inflammation
- Hip replacement failure
The Stryker Rejuvenate System was approved in 2008 for sale by Stryker. The faulty metal on metal hip implant device was designed to promote quicker installation with varieties of component designs. The necks are composed with heavy metal cobalt and chromium. The Rejuvenate and ABG II devices were designed to employ a two-piece modular neck and stem for enhanced flexibility and stability. According to Stryker, this design allows surgeons to choose a range of femoral neck and stem combinations and make it unique to each patient. However, the results of these recalled hip implants have been devastating. Components of the recalled hip implant contain metal and this can lead to fretting. This can then send particles into the patient’s tissues and even bloodstream.
Those patients who have any problems associated with Styker’s Rejuvenate and ABG II modular-neck stems may be entitled to compensation. Our Hip Implant Recall Lawyers are offering free personal injury lawsuit consultation to victims affected by Stryker’s faulty hip implants. If you or someone you love has been affected by Styker’s Rejuvenate and ABG II modular-neck stems, please contact our Hip Implant Recall Lawyers.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.