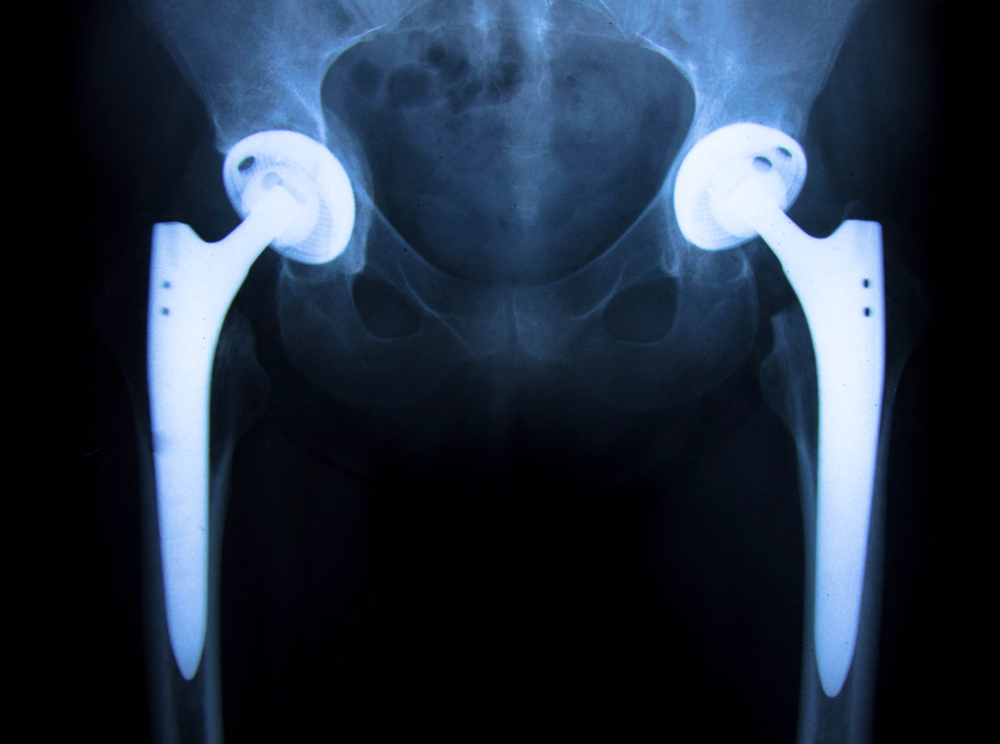
The FDA in April of 2016 updated its website regarding recommendations about soft tissue imaging and metal ion testing.
If you have been following the guidelines and information regarding the harms that have been documented as a result of the toxicity of the metal ions caused by the alloy comprised of cobalt and  chromium, you would see that the regulatory agencies and medical community are playing catch up as surgeons are seeing the harms and damage caused by these particular recalled components. This specifically applies to the Stryker Rejuvenate and Stryker ABG II hip implant systems (among others like the DePuy ASR hip implant system). Many surgeons are stating “that if you have one of these modular devices it’s not a matter of if the device will fail but rather when the device will fail.”
chromium, you would see that the regulatory agencies and medical community are playing catch up as surgeons are seeing the harms and damage caused by these particular recalled components. This specifically applies to the Stryker Rejuvenate and Stryker ABG II hip implant systems (among others like the DePuy ASR hip implant system). Many surgeons are stating “that if you have one of these modular devices it’s not a matter of if the device will fail but rather when the device will fail.”
In pursuing claims of patients that have these components, the hip implant recall lawyers at Childers, Schlueter & Smith have examined the records and counseled hundreds of patients in the process. We have encouraged patients to see their surgeon and/or have their orthopedic surgeon refer them to a qualified revision surgeon familiar with the failure of these devices. Unfortunately many surgeons that implanted these devices are not the surgeons that are qualified to remove them. In fact, many implant surgeons do not like to perform revision procedures given the time and complexity of the same. Be sure to ask your surgeon this very question and become more informed about your plan of care and treatment.
Our suggestion given all we have learned over the last 5 years working on recalled hip implants: “Be proactive and not reactive—do not wait for the pain or symptoms of loosening” says firm partner Richard R. Schlueter.
 In many patients that we represent, the patient does not experience pain or symptoms until significant damage to bone and tissue is in advanced stages. If you are one of the unlucky patients that have significant damage to your soft tissue, this amongst other complications, can lead to damage or loss of abductors that compromise future mobility, stability, and/or range of motion after undergoing a revision surgery to replace the components. Prevention is the key and sadly, patients need to be aware of this-especially those implanted with a Stryker Rejuvenate; Stryker ABG II; DePuy ASR; or a metal-on-metal DePuy Pinnacle hip implant system
In many patients that we represent, the patient does not experience pain or symptoms until significant damage to bone and tissue is in advanced stages. If you are one of the unlucky patients that have significant damage to your soft tissue, this amongst other complications, can lead to damage or loss of abductors that compromise future mobility, stability, and/or range of motion after undergoing a revision surgery to replace the components. Prevention is the key and sadly, patients need to be aware of this-especially those implanted with a Stryker Rejuvenate; Stryker ABG II; DePuy ASR; or a metal-on-metal DePuy Pinnacle hip implant system
Recommendations to consider initially if you have just learned that you have one of these devices:
- Contact your Doctor and schedule an appointment. Ask your doctor his familiarity with the failure rate of this device and whether he will treat you if it fails. If not, is he willing to refer you to a revision/repair surgery specialist if and when needed.
- Insist on a Metal Ion testing to check your levels of cobalt and chromium—(high levels are known to kill and damage soft tissue and eventually bone). Establish a baseline so in your next follow up, you can see if your metal ions levels are trending higher or remain stable.
- Consider on a MARS MRI even if you do not have symptoms and/or your physician is on the fence about it because you do not have identified symptoms. Ask the surgeon, “If it was you what would you do?” Getting a baseline is the best practice as you get monitored to see fluid buildup, inflammation and tissue destruction that is usually a lengthy and gradual process. Damage to soft tissue cannot be seen by x-ray only bone loss can.
- Make a reimbursement claim with Broadspire through your lawyer that will do so without charge or percentage of reimbursement on any out of pocket expenses that you incur for monitoring and/or costs associated with revision –including lost wages.
- Make a civil claim against the manufacturer for the substantial harms and damages associated with the need to be monitored and likelihood of additional surgery. The previous master settlement in the Stryker Hip Implant Recall [MDL 2441] does not cover patients that have surgery or testing after November 3, 2014. This claim must be made timely and within the statute of limitations of the resident state of the affected patient. Time is of the essence.
Individual considerations of the harms caused to each patient must be evaluated including the possible uncertainty of the longstanding effects of metallosis, cobalt and chromium. Each represented client has claims that are different and are evaluated individually. The hip implant recall lawyers at Childers, Schlueter & Smith, LLC evaluate each hip implant case on an individual basis. (We do not file these cases as part of a Class Action They are way too serious for that treatment).
Either Stryker will agree to an appropriate dollar value of your claim or the individual client’s claim will be vigorously pursued. In the estimation of Childers Schlueter and Smith, LLC the harms and  value of each timely prosecuted claim will be substantial. Childers Schlueter and Smith, LLC serves on the Plaintiff’s Steering committee by appointment of the Honorable Judge Donovan Frank in the consolidated national proceedings MDL 2441 in the U.S. District Court of Minnesota. (The PSC is the national leadership of a given litigation that serves to protect and promote the rights of all plaintiffs involved in addition to their own specific and individual clients).
value of each timely prosecuted claim will be substantial. Childers Schlueter and Smith, LLC serves on the Plaintiff’s Steering committee by appointment of the Honorable Judge Donovan Frank in the consolidated national proceedings MDL 2441 in the U.S. District Court of Minnesota. (The PSC is the national leadership of a given litigation that serves to protect and promote the rights of all plaintiffs involved in addition to their own specific and individual clients).
If you want help or just the ability to become better educated on a recalled hip implant system, call us and ask to speak to one of the hip implant recall attorneys at our firm. All calls are confidential and all initial consults are free of charge. You can also contact us via our firm website at www.CSSFIRM.com for more information
No matter what you decide, take care of yourself and do what is best for you.