CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
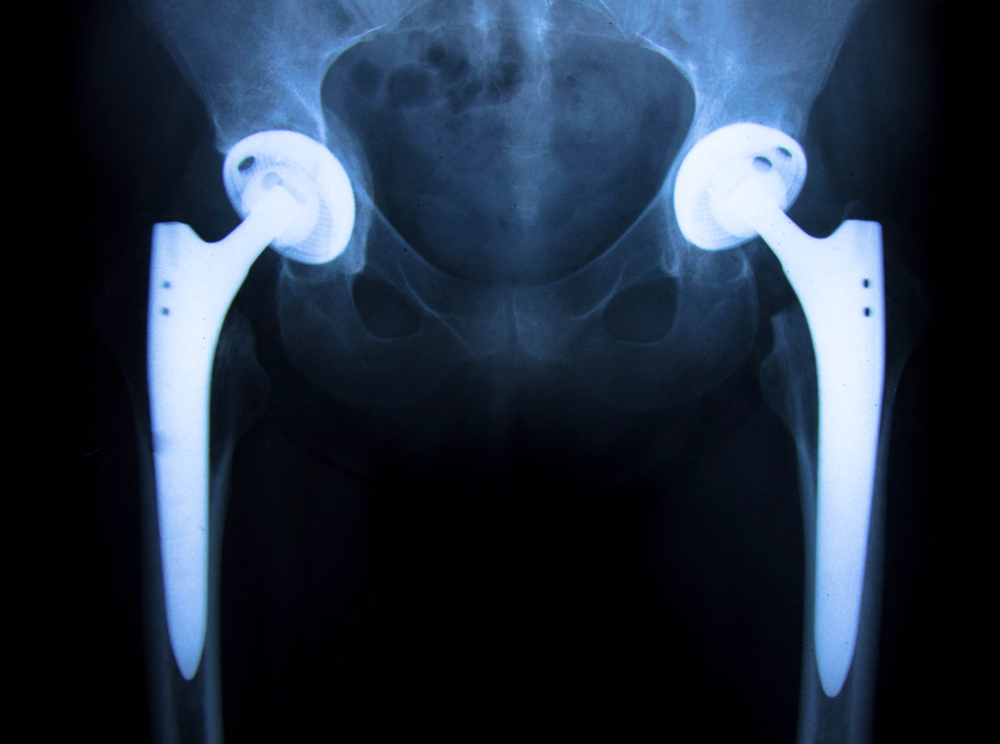
A U.S. District Court Judge recently ruled to allow a Stryker hip implant lawsuit to continue after she dismissed two of the plaintiff’s claims, but allowed a claim regarding manufacturing defect to stand. In the lawsuit, the plaintiff alleged that he had suffered injuries and pain and was forced to undergo revision surgery after his Stryker Gamma3 Nail hip implant system failed.
Although the judge dismissed the strict liability design-defect and negligence claims against Stryker, the lawsuit’s strict liability manufacturing defect, breach of warranty, and loss of consortium claims will stand. The Gamma3 system is a metal on metal (MoM) hip implant, devices that have come under fire for causing a number of severe complications in patients.
Risks of Metal-on-Metal Hip Implants
All artificial hip implants carry risks including wear and tear of the component material, and MoM implants have unique risks in addition to the general risks of all hip implants:
- When the metal ball and the metal cup slide against each other during walking or running, tiny metal particles are released into the body.
- Wear and corrosion at the connection between the metal ball and taper of the step may occur.
- Metal ions (cobalt and chromium) from the metal implant or metal particles may enter the bloodstream.
- Adverse local tissue reaction, which overs over time when the metal particles around the implants cause damage to the surrounding bone and/or tissue, resulting in pain, implant loosening, device failure, and the need for revision surgery.
According to the U.S. Food and Drug Administration (FDA), patients with a MoM hip implant may exhibit certain symptoms or illnesses elsewhere in the body. These include:
- General skin rash
- Cardiomyopathy
- Neurological changes including auditory or visual impairments
- Psychological status change, including depression or cognitive impairment
- Renal function impairment
- Thyroid dysfunction, including neck discomfort, fatigue, weight gain or feeling cold
In April 2015, the FDA recommended that asymptomatic patients with MoM hip implants continue to follow-up with their orthopaedic surgeon every 1 to 2 years to monitor for early signs of change in hip status.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
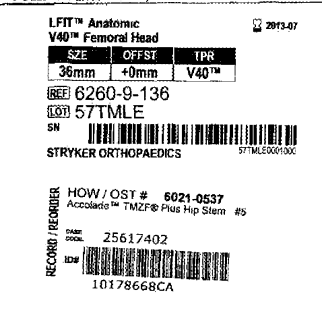
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.
Stryker’s track record includes multiple recalls and lawsuits involving defective hip implants—and recent reports suggest continued failures in other device components, raising serious safety concerns.