Stryker Medical has elected to use the company Broadspire Services to help manage claims and recalls of the defective Rejuvenate and ABG II hip systems. Broadspire representatives are allegedly approaching Stryker hip lawsuit clients and offering lower forms of compensation to them, including an immediate sum of money in exchange for an agreement not to sue the company and a release to forever waive all their legal rights no matter what the future may hold. Broadspire is hoping to save the company millions of dollars at the expense of affected consumers and patients.
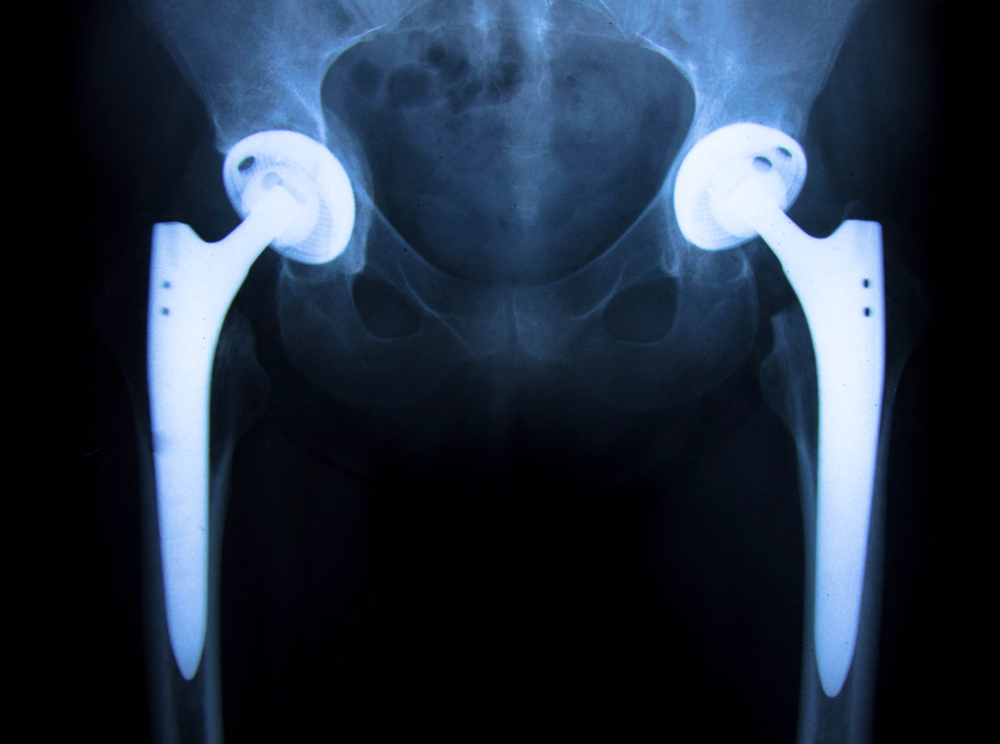
Stryker is one of several manufacturers of the implants that were recalled by the FDA in July 2012. The recall was initiated due to potential risks with the hip replacement systems, including fretting and/or corrosion at the modular-neck junction of the system. The Stryker system reportedly had a high early failure rate that resulted in the need for patients to undergo revision surgeries to replace the devices more often than with other hip replacement systems. For more information on the Stryker Hip Implant Recall-See our previous post on the “Top 10 Things All Stryker Hip Implant Patients Need To Know”
FDA Approval
Stryker received approval from the FDA to sell the Rejuvenate system in the U.S. in 2008, and the ABG II system was approved for sale in 2009. Both were approved through the FDA’s controversial 510(K) medical device (“short-cut”) approval process, which allows manufacturers to market and sell medical devices without any pre-market testing or clinical trials, provided the manufacturer can prove that the device is substantially similar to a device already on the market. As a result, rather than testing artificial hips before they were marketed and sold, Stryker only conducted post-market surveillance.
Complications
Known complications associated with the Rejuvenate and ABG II systems include:
- Blood toxicity from metal debris
- Misalignment and loosening of system components
- Intensified pain
- Pseudo tumors
- Metallosis
- Tissue and bone necrosis
The treatment required to correct these complications is often a very complicated revision surgery in which the entire femoral system is removed and replaced with another larger metal stem. Because the stem is inserted down the center of the femur bone, removal is difficult and often results in fractures to the femur, which then has to be repaired with more metal hardware. The recovery process is often long and complicated, with a high potential for permanent loss of hip function and mobility.
The Stryker hip implant lawyers at Childers, Schlueter & Smith continue to review and investigate new Rejuvenate and ABG II hip implants on a daily basis. If you have received a call from a Broadspire representative and have questions about your potential claim call us for a free initial evaluation and review.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
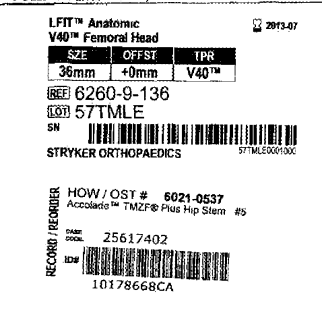
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.