The FDA’s rule to make generic drugmakers accountable for inadequate safety labeling is under attack from the generics industry, and we need your help pushing the agency ahead. Click on this link to help right now: Holding Generic Drug Makers Accountable
The backstory: In the Mensing decision in 2011, SCOTUS said that a failure-to-warn suit against a generic drugmaker was preempted, which left generics makers unaccountable. In 2013, the FDA proposed a rule to enable generics manufacturers to independently update their safety labeling in response to ne…w evidence of harms, and be taken to court if their warnings are inadequate.
But the rule has been delayed, and most recently the FDA reopened the public comment period on the rule. They also held a public meeting in March on the rule, and individuals harmed by generic drugs told FDA their compelling stories and spoke in favor of finalizing the rule.
An FDA official acknowledged our previous petition on the issue, which garnered roughly 20,000 signatures. We want to beat that number this time to show the agency the support it has to move forward.
Holding Generic Drug Makers Accountable
Please help us today by signing the petition, sharing it on Facebook, and promoting this positive change that will help make our families and communities safer.
Other Dangerous Drugs News
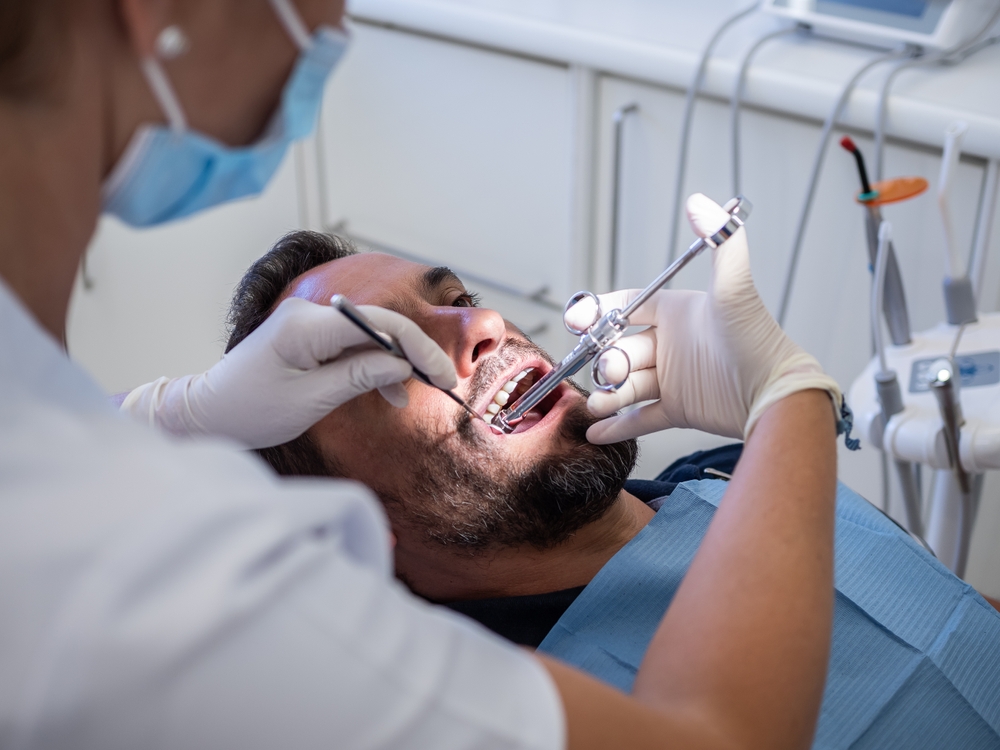
While Suboxone helps fight opioid addiction, it’s now at the center of a growing legal battle over severe, preventable dental harm not disclosed to patients for years.
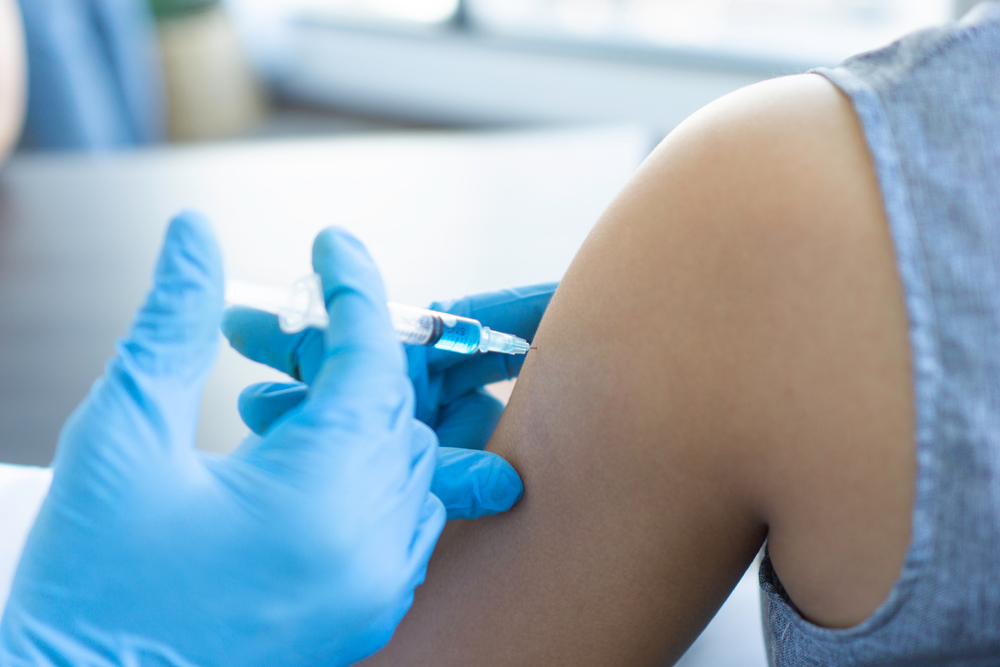
Women across the U.S. are filing lawsuits against Pfizer after a major study linked long-term use of Depo-Provera to a fivefold increase in the risk of developing brain tumors known as meningiomas.
Women diagnosed with meningiomas after using Depo-Provera may be eligible for financial compensation, as lawsuits claim Pfizer failed to warn about the potential risk of brain tumors.
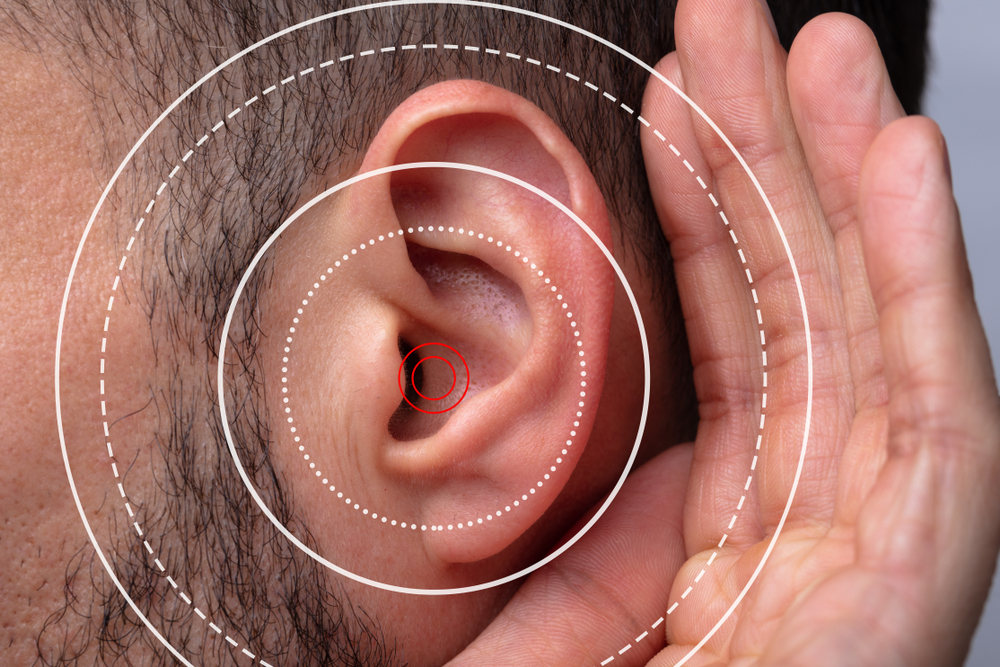
Patients who took Tepezza for thyroid eye disease and suffered hearing loss—including deafness—are now suing the manufacturer for failing to warn of these devastating side effects.
Despite growing evidence that Tepezza may cause hearing loss or tinnitus, the drug remains on the market—and lawsuits are mounting against its manufacturer.