A superbug outbreak at UCLA has prompted the family of a patient who died in January 2015 to bring suit against a medical device maker, Olympus Corp. of the Americas, for wrongful death.
The family of 41-year-old Silvia Patricia Aroche filed the lawsuit on March 13, 2015 in Los Angeles Superior Court. According to court documents, the woman was exposed to the deadly CRE bacteria from a contaminated Olympus duodenoscope at UCLA’s Ronald Reagan Medical Center while undergoing multiple procedures in December 2014.
CRE Bacteria
CRE stands for carbapenem-resistant Enterobacteriaceae, a bacteria that is highly resistant to antibiotics and is estimated to have the ability to kill up to 50 percent of infected patients, according to the Centers for Disease Control and Prevention (CDC).
Healthy people do not typically get CRE infections. Instead, they usually happen to patients in hospitals, nursing homes, and other healthcare settings, and those most at risk for contracting a CRE infection include those whose care requires certain devices, including:
- Ventilators
- Urinary catheters
- Intravenous catheters
Those who are taking long courses of certain antibiotics are also considered to be at risk for CRE.
Olympus Duodenoscopes
The Olympus duodenoscopes are typically used to perform a medical procedure known as endoscopic retrograde cholangiopancreatography (ERCP), during which a doctor threads the thin device down the patient’s throat to examine and treat conditions such as cancer, gallstones, and other digestive system issues. After the UCLA infestations, the U.S. Food and Drug Administration warned hospitals that the duodenoscopes are hard to rid of dangerous bacteria, even when following the manufacturers’ directions for reprocessing.
Five lawsuits have been filed against Olympus over contaminated Olympus duodenoscopes thus far. According to the company, seven patients in all were infected with the CRE superbug from scopes made by Olympus, leading to two deaths and exposing an estimated 179 other patients from October 3, 2014 to January 28, 2015.
Other Medical Devices News
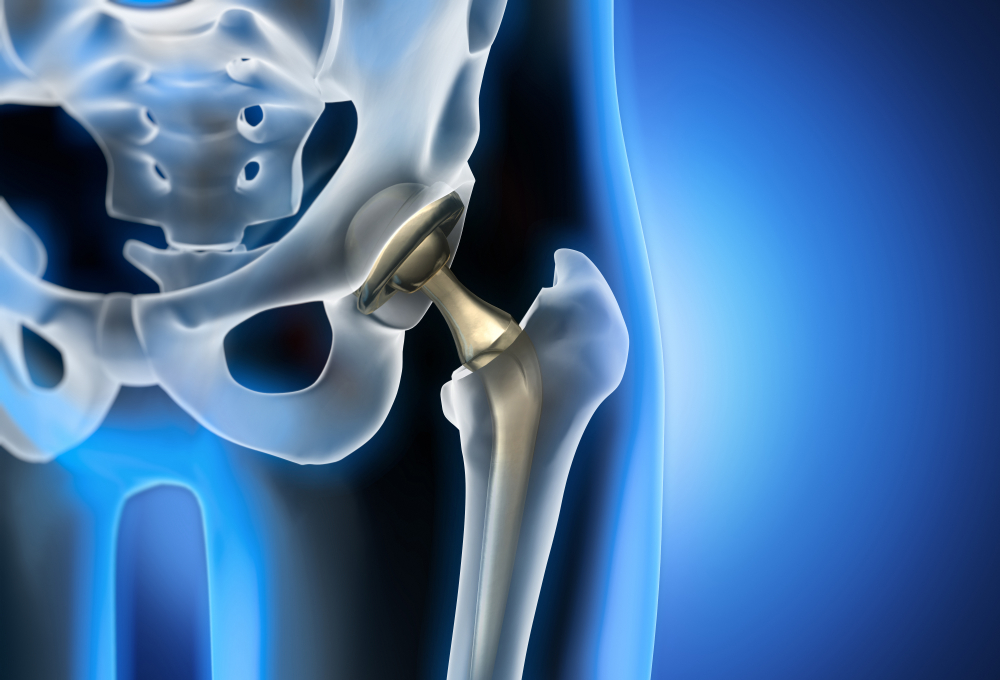
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
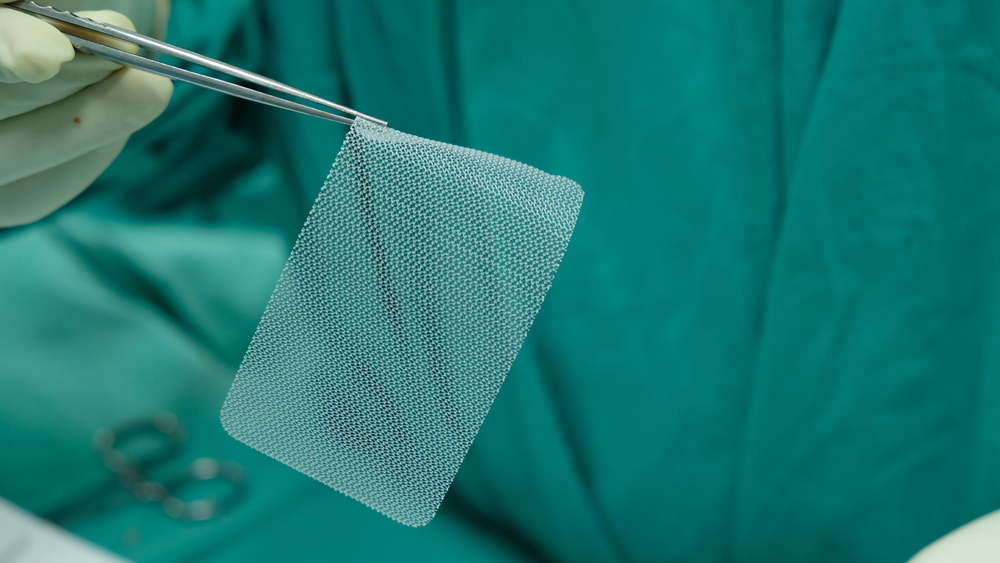
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
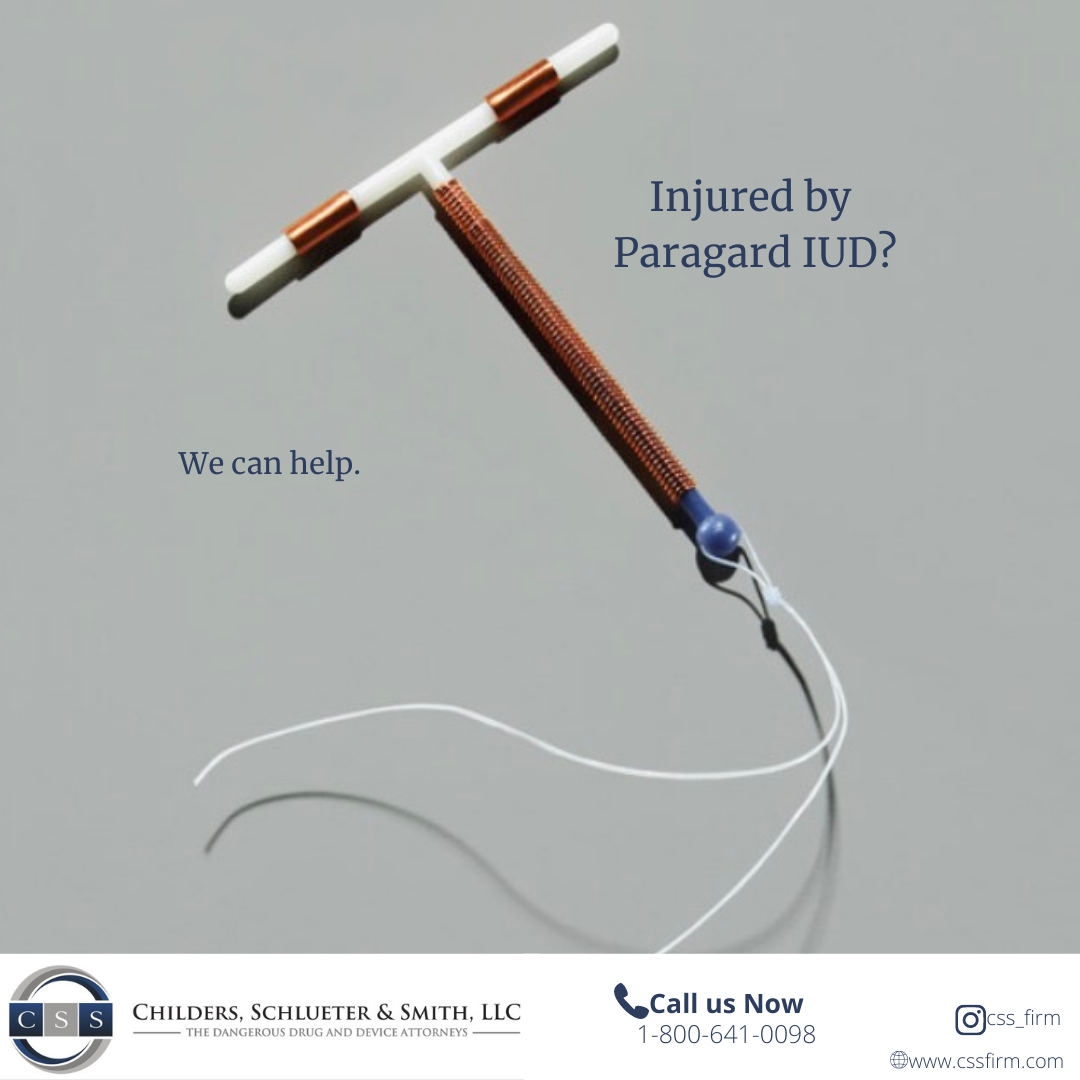
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.
A voluntary recall affects over 3.5 million Philips Respironics CPAP, BiPAP, and ventilator devices due to foam degradation risks, potentially endangering sleep apnea patients’ health and safety.
Philips Respironics has recalled certain CPAP, BiPAP, and ventilator devices due to degrading sound foam that may release harmful particles and chemicals, posing serious health risks to users.