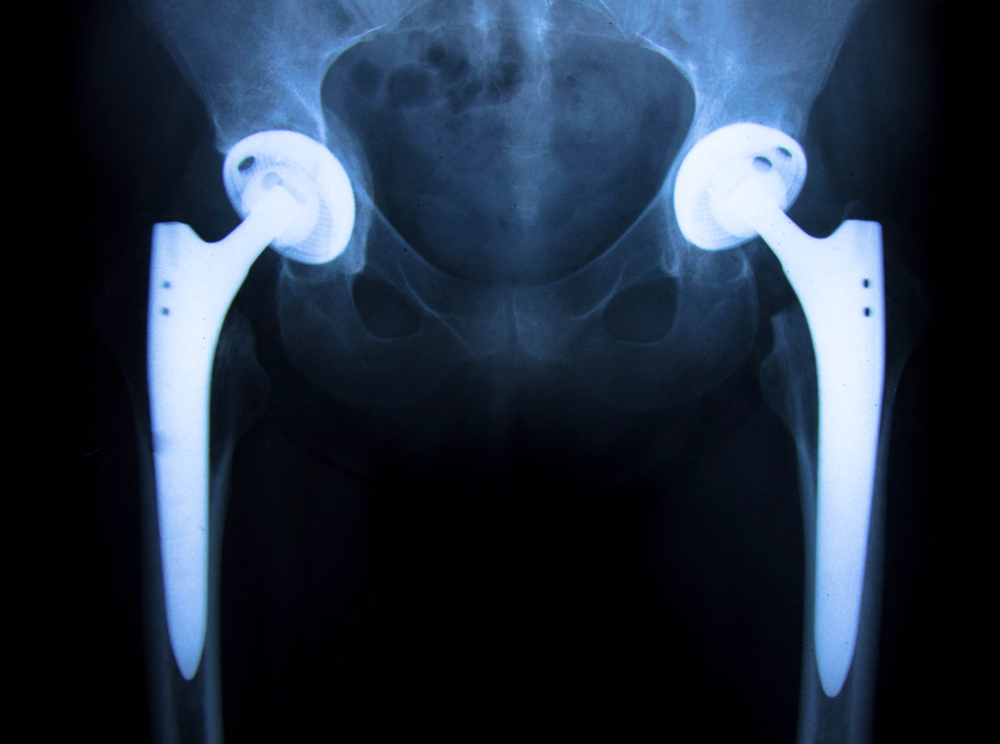
Judge Donovan W. Frank, presiding over the Stryker Hip Implant MDL in the U.S. District Court for the District of Minnesota, ordered the formation of leadership involving a Plaintiffs’ Steering Committee (PSC). Richard R. Schlueter of Childers, Schlueter & Smith has been appointed to the PSC and represents the only Georgia based law firm on the leadership panel. The Stryker MDL was established in June 2013, when a group of federal Stryker Rejuvenate, and ABG II lawsuits were transferred from federal courts around the country, to Judge Frank’s Court in Minnesota for consolidated pretrial discovery.
The Stryker Hip Implant Litigation has grown significantly to include hundreds of lawsuits brought by plaintiffs alleging that they have suffered injuries caused by the manufacturer’s recalled Rejuvenate and ABG II hip replacement implants. Childers Schlueter & Smith LLC was a leading firm in early filings against Stryker and continues to investigate new cases on behalf of patients nationwide. The total number of cases is expected to climb even higher as more than 20,000 of these devices were sold in the U.S. prior to the recall.
Since 2009, Childers, Schlueter & Smith LLC has been active in representation of harmed patients with faulty orthopedic devices and has since that time taken a leading role to protect consumers and patients on a nationwide basis. These devices include products manufactured by Stryker; DePuy Orthopedics; Wright Medical; Zimmer; and Biomet.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.