The U.S. District Court for the Northern District of Georgia agreed to the transfer of dozens of lawsuits filed over the Paragard T 380A intrauterine birth control device into a multidistrict litigation (MDL). Despite opposition from Teva Pharmaceuticals and other defendants in the case, the MDL was assigned to Judge Leigh Martin May.
Judge May recently appointed Andy Childers as Plaintiffs’ Liaison Counsel for MDL 2974, as well as appointing 22 other attorneys to the Plaintiffs’ Executive Committee and the Plaintiffs’ Steering Committee.
Instead of thousands of individual Paragard IUD lawsuits going through the court system, an MDL allows one judge to make all the pretrial decisions for all the combined cases. Ultimately, a few cases, known as bellwether cases, will be tried in court. How these cases are decided will likely affect settlement negotiations in the remaining cases. The Paragard litigation is not a class action (a process that combines all the cases into one large case). Individual lawsuits have been consolidated into an MDL, which is a legal mechanism intended to speed up pretrial proceedings. In short, the MDL streamlines the litigation process.
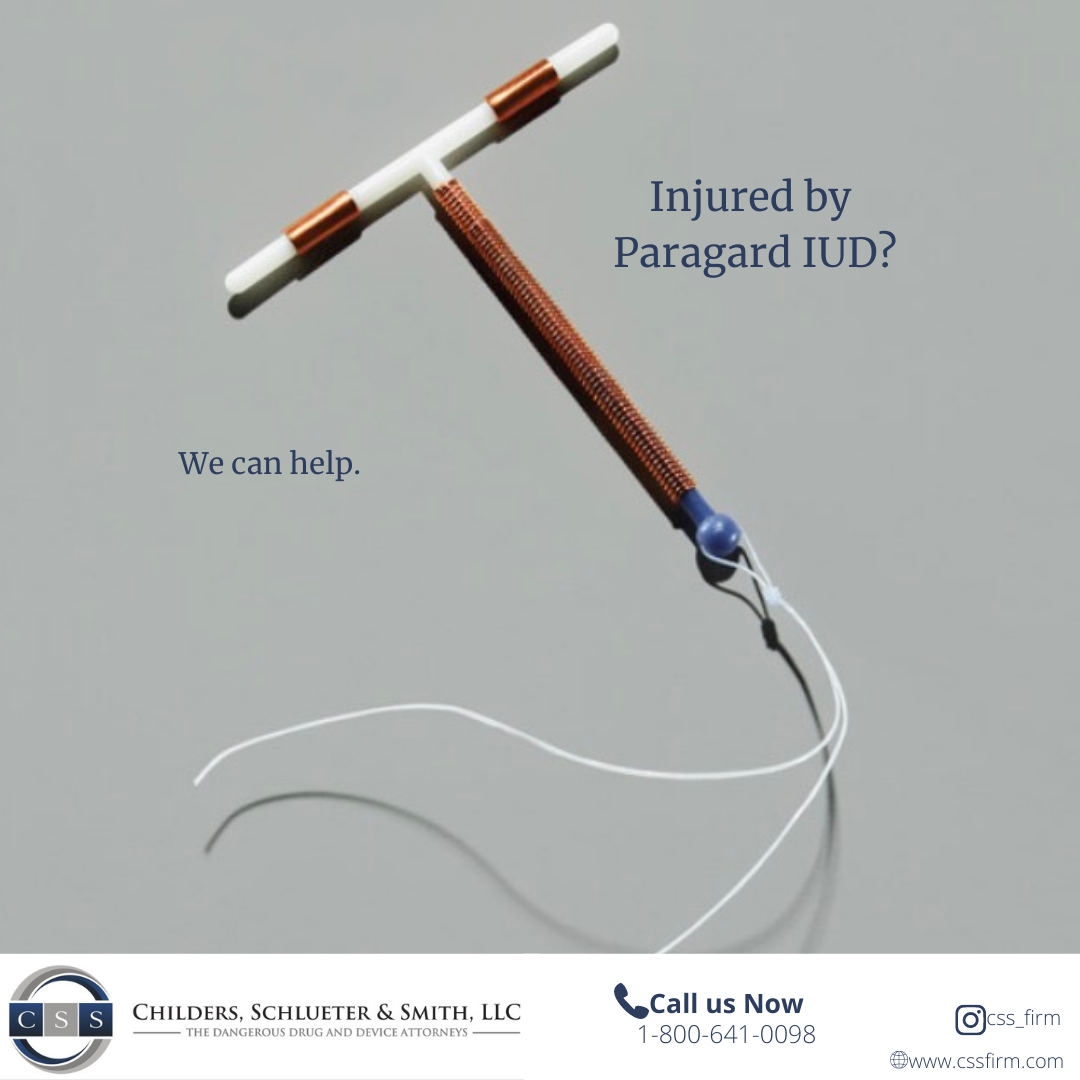
Plaintiffs claim that there was a risk of the device fracturing while in the body or upon removal and that Teva and the other manufacturers knowingly marketed the device as safe and easy to remove. The lawsuits allege that Paragard-related breakage injuries caused the plaintiffs to suffer inflammation, infection, scarring, organ damage, long-term infertility, and hysterectomy-related injuries.
What is Paragard?
Paragard, approved by the FDA in 1984, is an intrauterine device (IUD) used by women for long-term birth control. Teva Pharmaceuticals manufactured the Paragard IUD and marketed it as a small, hormone-free, flexible, and soft device that is over 99 percent effective in preventing pregnancy. For those wanting to avoid hormones, the device utilizes copper to produce an inflammatory reaction that is toxic to sperm and eggs. Paragard is the only copper IUD available in the U.S., and the company claims it can prevent pregnancy for up to 10 years after insertion.
The Paragard T 380A IUD remains on the market, despite lawsuit allegations that it is a dangerous and defective medical device. One batch of the device was recalled in 2019 due to sterility, not breakage, issues.
If you have experienced injuries caused by a Paragard IUD, give us a call for a free case consultation. If you would prefer, you can also submit a request online. Provide as much information you feel is necessary, click submit, and we will be in touch after review.
Other Paragard IUD Lawsuits News
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.
The Paragard IUD has been linked to painful injuries during removal—including broken fragments and embedded devices. Our firm is reviewing Paragard-related injury claims nationwide.
Women across the country are coming forward after suffering devastating injuries from broken Paragard IUDs—including uterine perforation, organ damage, and the need for hysterectomy.