Medtronic recently settled a lawsuit for $2.8 million, leading to a dismissal of charges but no admission of liability on the part of the company. The federal government filed the action on behalf of whistleblower Jason Nickell, a former Medtronic salesman who made upwards of $600,000 per year by selling Medtronic neuromodulation devices to physicians and hospitals, but quit his job over concerns about the way the devices were being promoted. Nickell will receive $602,000 under the whistleblower provisions of the False Claims Act.
Lawsuit Allegations
The questionable procedure to which Nickell objected is known as subcutaneous stimulation, Sub-Q, or subcutaneous peripheral nerve field stimulation, a use not approved by the FDA. According to court documents, Medtronic sales staff was directed to promote the off-label procedure by selling the neuromodulation device at extreme discounts to pain management doctors by promising them that they could make as much as $10,000 profit on each patient while adding only minutes to the procedure.
The Justice Department charged Medtronic of paying tens of thousands of dollars to doctors in 20 states to encourage the health care providers to use the device in an off-label manner. The company is also alleged to have told hospitals and surgery centers to bill Medicare for the unapproved procedure by using a billing code meant for FDA-approved use, and once paid a physician $1500 a day to let other doctors watch him perform the off-label procedure to train them how to do it.
About Medtronic
Medtronic has its principal offices in Ireland, its operational headquarters in Minneapolis, Minnesota, and is the world’s third largest medical device company, but has had its share of brushes with the law. Among them: In 2008, the company was ordered to pay $75 million to settle a whistleblower Medicare billing fraud case, and in 2014, it was ordered to pay over $1 billion to settle a patent litigation case with Edwards Lifesciences and agreed to pay $22 million to settle cases related to the controversial bone-growth product Infuse, which has been the subject of many lawsuits.
Other Medical Devices News
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
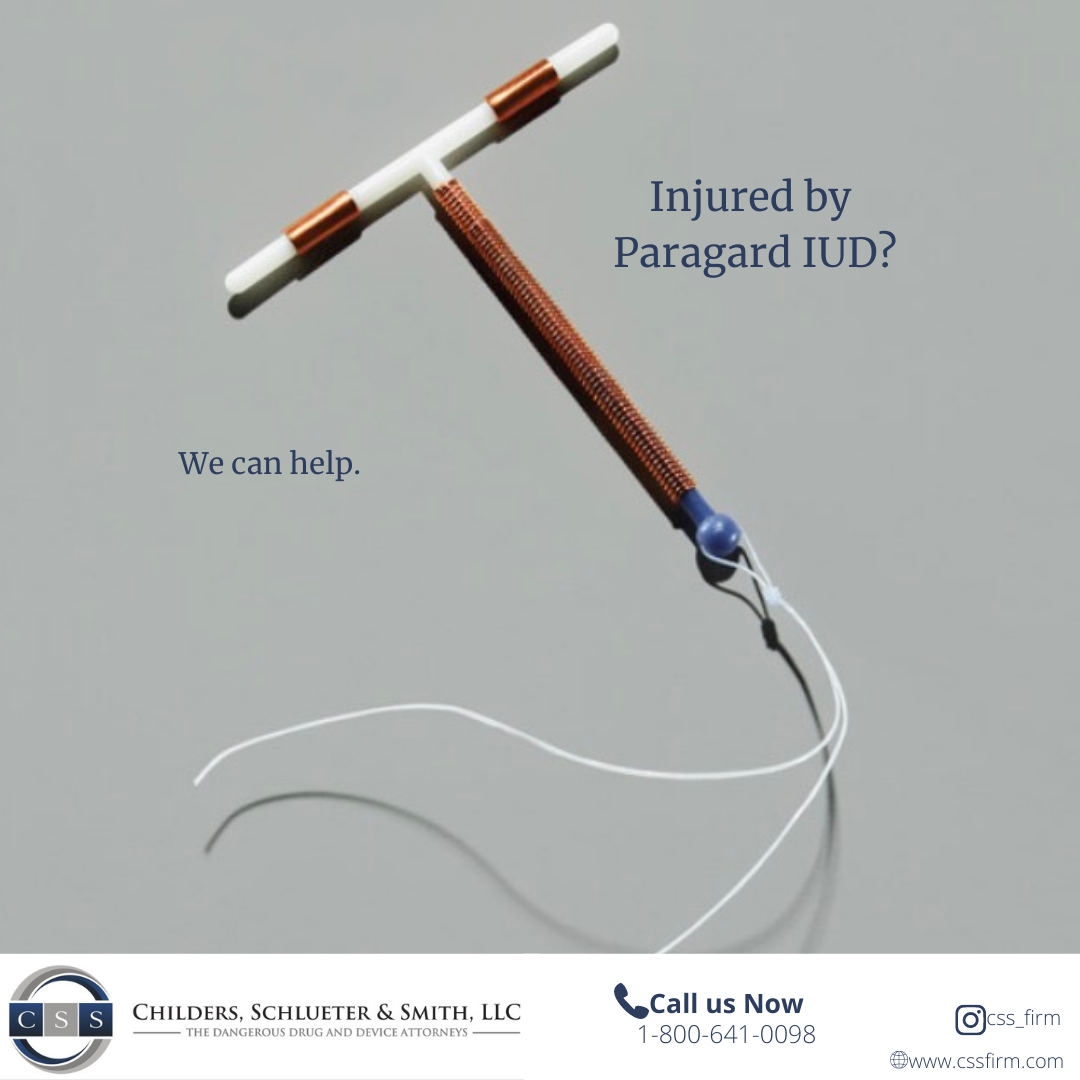
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.