Medtronic Paradigm insulin pumps are used by diabetics to keep their insulin levels stable, but when these pumps malfunction, dangerous injuries can result. As a result, an Urgent Medical Device Recall has been issued. 
What are Medtronic Paradigm Pumps?
Medtronic Paradigm insulin pumps are intended to deliver specific amounts of insulin to insulin-dependent diabetics. The insulin is dispensed from the pump’s reservoir, travels through the infusion sets and is delivered to the user via a needle inserted into the patient’s body.
The ability of this pump system to function properly depends on a component that connects the pump to the infusion sets. This “connector” contains vents that allow the air to flow through the pump system, but if the component becomes wet, liquid can clog the vents, triggering an over- or under-delivery of insulin. Depending upon the patient’s sensitivity to insulin, even a slight difference in the amount delivered can result in hyper- or hypoglycemia, leading to a number of severe side effects, including:
- Anxiety
- Confusion
- Cold sweats
- Trembling
- Fatigue
- Hunger
- Irritability
- Loss of consciousness
- Seizures
- Death
In September 2017, Medtronic announced a voluntary recall of certain lots of infusion sets used with all models of Medtronic insulin pumps connected to the over-delivery of insulin. The Paradigm insulin pumps are designed and manufactured by Medtronic MiniMed, Inc., a subsidiary of Medtronic, Inc.
Customers in the United States (U.S.) can determine if they have recalled infusion sets by visiting https://checklots.medtronicdiabetes.com.
Medtronic Paradigm Pump Lawsuits
Patients who have sustained serious injuries because of issues with Medtronic Paradigm insulin pumps may be eligible to file lawsuits to recover damages. In March 2018, a wrongful death lawsuit was filed over issues with a Medtronic MiniMed insulin pump. The device allegedly malfunctioned and pumped an entire week’s worth of insulin in a Mississippi woman in one night and she suffered a fatal stroke due to severe hypoglycemia. The device has been linked to more than a dozen deaths in recent years, according to a Minneapolis Star Tribune report.
Please Note: This article is intended for educational purposes only. CSS Firm is not currently accepting Metformin/Medtronic claims as research is still developing.
Other Medical Devices News
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
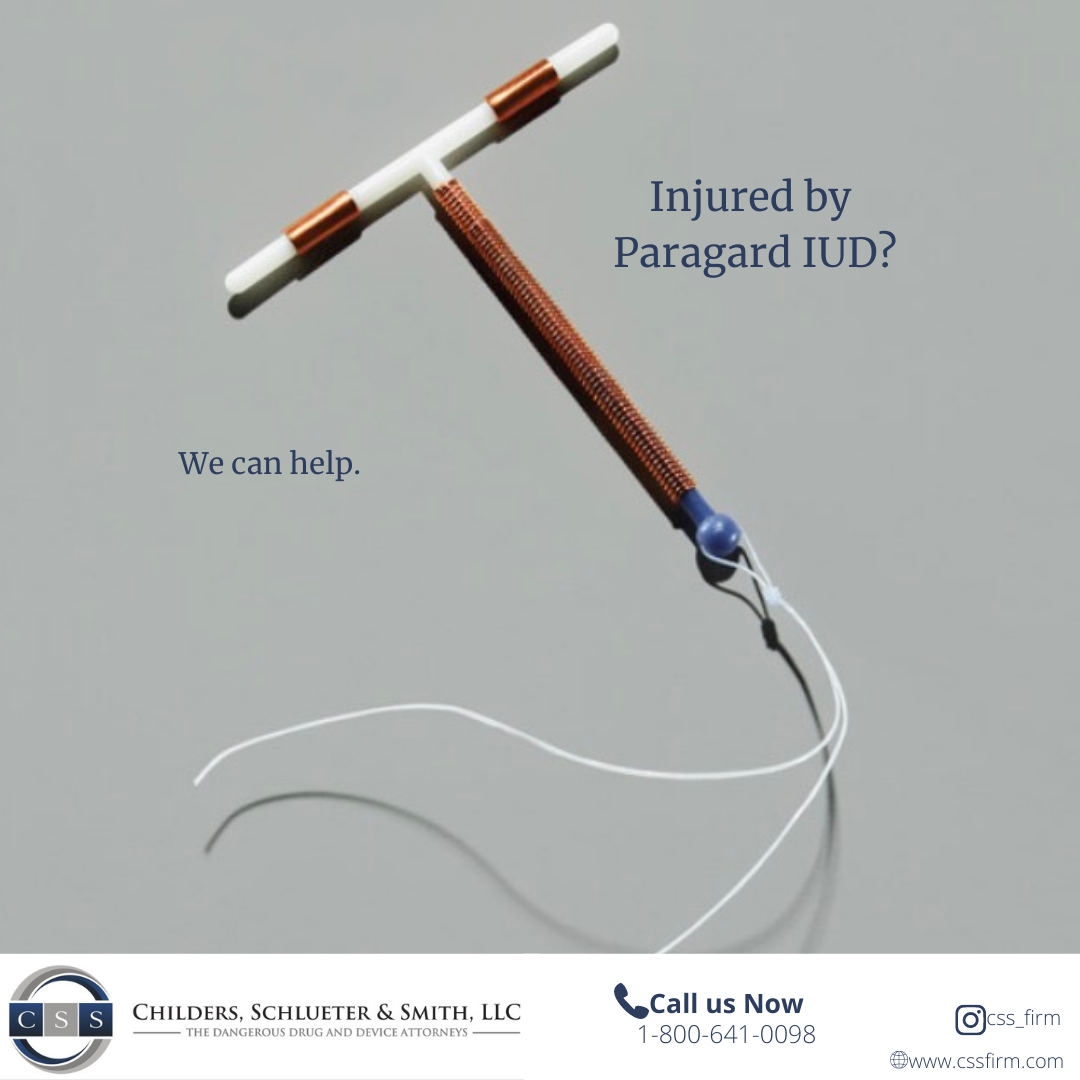
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.
A voluntary recall affects over 3.5 million Philips Respironics CPAP, BiPAP, and ventilator devices due to foam degradation risks, potentially endangering sleep apnea patients’ health and safety.
Philips Respironics has recalled certain CPAP, BiPAP, and ventilator devices due to degrading sound foam that may release harmful particles and chemicals, posing serious health risks to users.