Please Note: CSS Firm is no longer accepting or investigating new DePuy Attune knee implant claims at this time. This article is for educational purposes only.
Breaking News-Attune Knee Implant Update
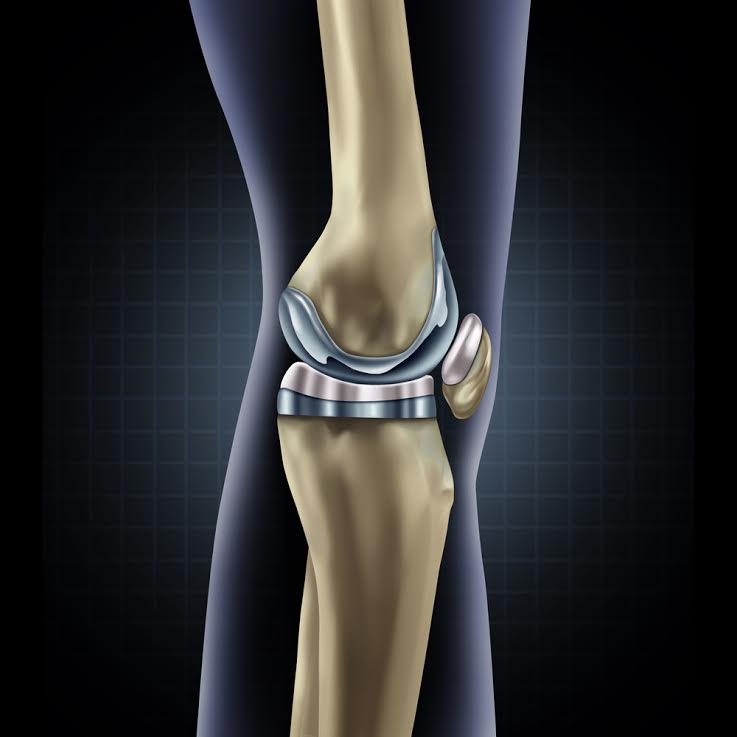
The first DePuy Attune Knee lawsuit has been filed in Alabama claiming a significant failure of the fixed bearing tibial base plate in the DePuy Attune cemented knee. It is believed that hundreds of additional lawsuits will follow in the coming months and years ahead. Case studies and adverse event reports, reported to the FDA claim the tibial base plate of the DePuy Attune fails to adhere to the cement mantle and comes out clean or with little adherence or attachment to the cement interface. Is this the tip if the Iceberg?
in the DePuy Attune cemented knee. It is believed that hundreds of additional lawsuits will follow in the coming months and years ahead. Case studies and adverse event reports, reported to the FDA claim the tibial base plate of the DePuy Attune fails to adhere to the cement mantle and comes out clean or with little adherence or attachment to the cement interface. Is this the tip if the Iceberg?
It is not known how many Attune patients may be affected but it is believed more than a hundred thousand patients were implanted with the DePuy Attune Knee. Our office has already discussed and reviewed records of premature failures and complications from the Attune Knee replacement system that have been revised because of tibial loosening events.
 It is difficult to diagnose the loosening of the device. However, many surgeons are surprised to find little to no bonding of cement attachment to the underside of the tibial tray to the cement mantle. In June, The Journal of of Knee Surgery published “Unusually High Rate of Early Failure Of Tibial Component in Attune Total Knee Arthroplasty System at Implant- Cement Interface.” Knee Surg 2017; 30(05): 435-439
It is difficult to diagnose the loosening of the device. However, many surgeons are surprised to find little to no bonding of cement attachment to the underside of the tibial tray to the cement mantle. In June, The Journal of of Knee Surgery published “Unusually High Rate of Early Failure Of Tibial Component in Attune Total Knee Arthroplasty System at Implant- Cement Interface.” Knee Surg 2017; 30(05): 435-439
The author of the article Peter M. Bonutti, MD writes “ We have encountered a High rate of debonding of tibial implant- cement interface. In addition, multiple reports have been filed in Manufacturer and User Facility Device Experience database (MAUDE) with the same mechanism of failure.”
DePuy counters the Bonutti article contains inaccuracies and is limited in a white paper it published following the publication of the Bonutti article. But we expect much more information to come to light about the DePuy Attune Knee in the months ahead.
DePuy has now launched another version of the Attune Knee tibial base plate known as the “Attune S+” Based upon information and belief, many surgeons that use the current Attune product have not been informed or made aware of this new design. Notably the surface of the underside of the tibial plate has a different grit and cut outs on the underside to improve the interface of the cementing to the tibia.
and belief, many surgeons that use the current Attune product have not been informed or made aware of this new design. Notably the surface of the underside of the tibial plate has a different grit and cut outs on the underside to improve the interface of the cementing to the tibia.
This begs the question, If the current tibial base plate is not problematic in its loosening, why redesign it with enhanced features of cement bonding with the Attune S+? Time will tell, but we think we already know why based on our research and investigations. Hopefully, DePuy will be responsible in addressing the ongoing problems with the earlier version of the Attune knee product and will start educating physicians to the same in order to promote patient safety and monitoring. If this knee system has a latent failure as suggested by Bonutti, DePuy may need to initiate a global recall of the DePuy Attune product in the future to avoid its continued use and damage in other patients. To date however, DePuy has refused to take those steps with most doctors and has yet to even hint at a Attune Recall claiming the product preforms as expected.
If you have questions about your DePuy Attune Knee replacement, call the DePuy Attune Knee Implant Lawyers at Childers, Schlueter & Smith. We are currently investigating the Attune knee and have lots of information about it already. All initial consults are free of charge and completely confidential.
Email at: Info@cssfirmllcdev.wpenginepowered.com
Disclaimer: Our description of your legal rights is not intended to imply that any product is defective per se. That can only be determined through a case-specific investigation and review. DePuy is the registered trademark of DePuy Orthopaedics and of Johnson & Johnson Company. The use of this trademark is solely for product identification and informational purposes. DePuy Orthopaedics and Johnson & Johnson Company or any of its companies are not affiliated with this website. Both companies and their recalled ASR Hip Implant products have no affiliation with Childers, Schlueter & Smith, LLC. Nothing on this site has been authorized or approved by DePuy Orthopaedics or Johnson & Johnson Company. To be clear, Childers, Schlueter & Smith, LLC is completely adverse to said companies and the opinions contained herein are those of the Firm alone based on their investigations, client stories and review.
Other DePuy Attune Knee Implant Lawsuits News
Reports of premature failure in DePuy Attune Knee Systems have raised concerns, with patients experiencing pain, instability, and revision surgeries much sooner than expected after implantation.