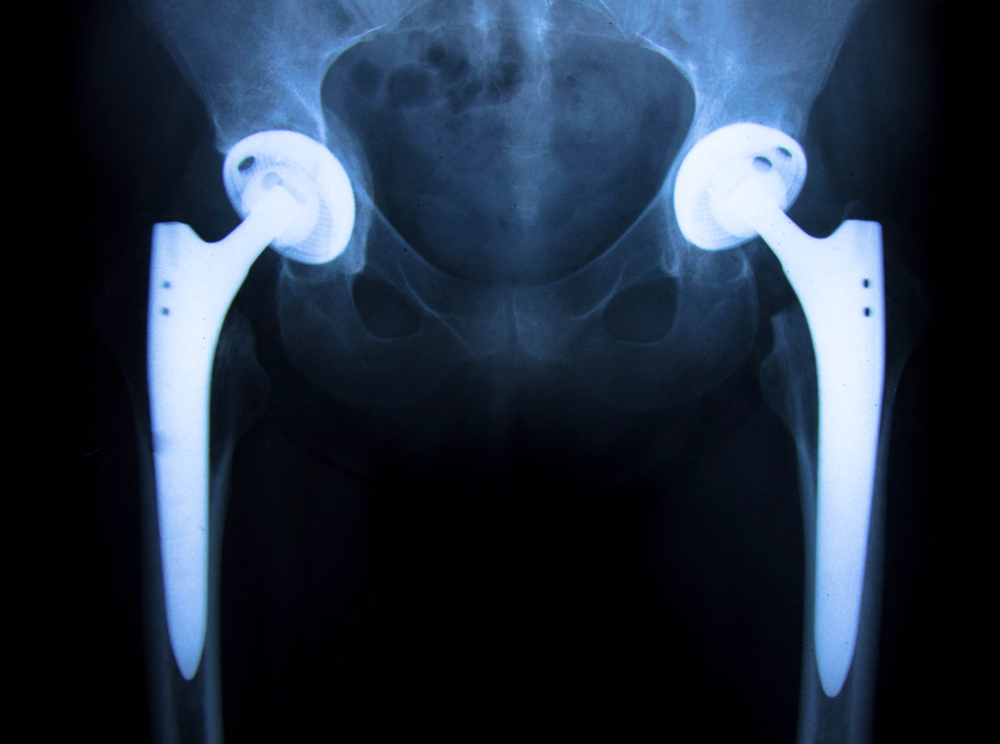
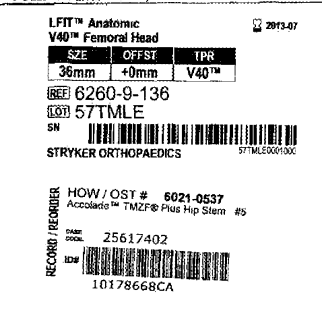
The Stryker LFIT Anatomic Cobalt Chromium (CoCr) V40 femoral head had a limited voluntary recall in August, 2016, which can be viewed here. Recalled lots included femoral heads diameter and offsets of 36+5, 40+4, 40+8, 40+12, 44+4, 44+8, and 44+12 (head diameter, neck offset). Other remaining femoral head sizes have not been recalled. One of the reported hazards noted was disassociation of the femoral head-stem and Gross Trunnion Failure (GTF). Absent from the “Urgent Medical Device Recall Notification” is the mention of investigating or reported events of head/taper corrosion at the interface with the Titanium stem. There are many reports of this occurring with the FDA Maude Database.
The Stryker LFIT Anatomic Cobalt Chromium (CoCr) V40 femoral head had a limited voluntary recall in August, 2016, which can be viewed here. Recalled lots included femoral heads diameter and offsets of 36+5, 40+4, 40+8, 40+12, 44+4, 44+8, and 44+12 (head diameter, neck offset). Other remaining femoral head sizes have not been recalled. One of the reported hazards noted was disassociation of the femoral head-stem and Gross Trunnion Failure (GTF). Absent from the “Urgent Medical Device Recall Notification” is the mention of investigating or reported events of head/taper corrosion at the interface with the Titanium stem. There are many reports of this occurring with the FDA Maude Database.
Further, many of the clients that we represent have significant corrosion discovered at the head-neck junction of the Stryker stem. Almost always, this is associated with the earlier version of the Accolade Stem known as Accolade TMZF, or also commonly referred to as Accolade 1. Its known that the TMZF alloy is proprietary to Stryker. Stryker changed the Titanium alloy in a new design known as the Accolade 2 with a standardized cross industry alloy of Titanium, but the earlier version of the TMZF Accolade is still available. Could this be part of the problem? It’s believed with more patients coming forward in filing claims, and case studies investigated by revising surgeons, the truth will come out of the risk associated with failures of the use of the the Stryker LFIT Anatomic Cobalt Chromium (CoCr) V40 femoral head lots that have not been recalled, but have patients at risk for head /taper corrosion.
Given our work on previous Stryker Hip Implant Recalls, we know a lot about Stryker and their products. For this reason and others, Childers Schlueter & Smith continues to investigate and pursue Stryker LFIT V40 Femoral head claims associated with the Accolade TMZF stem. If you have questions about your hip implant system, please call our Stryker Hip Implant Lawyers or send us a confidential message at cssfirmllcdev.wpenginepowered.com or email us at intake@cssfirmllcdev.wpenginepowered.com.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Stryker’s track record includes multiple recalls and lawsuits involving defective hip implants—and recent reports suggest continued failures in other device components, raising serious safety concerns.