While there are many innovative medical devices that have saved lives, the Food and Drug Administration’s (FDA) regulation processes are often times rushed, allowing defective products to slip through the cracks and to the public. In an original Netflix documentary, The Bleeding Edge, viewers learn the distinct difference between two regulation processes: premarket notification 510(k) and pre-market approval (PMA).
FDA commissioner from 1990-1997, Dr. David Kessler gave an interview, arguing that the FDA is the most important consumer protection agency in the world. Dr. Kessler also admits that the system is indeed broken:
“The FDA does an incredible job with the vast majority of products. The problem we have, is when it comes to medical devices, we built a system that doesn’t work.”
The medical device industry has certainly evolved since the regulation processes were put into place when the FDA assumed control over medical devices with the Medical Device Amendments of 1976. Devices on the market prior to that amendments were “grand-fathered” in to stay on the market without testing.
Profit over People?
The expense of testing any product for human consumption can rack up millions, a necessary obstacle to ensure the safety of consumers. The 510(k) process is used 98% of the time, whereas PMA, the much more extensive of the two, is used 2% of the time. The 510(k) process only requires manufacturers prove their product is significantly similar to products presently on the market, regardless of whether or not its predecessor had been recalled.
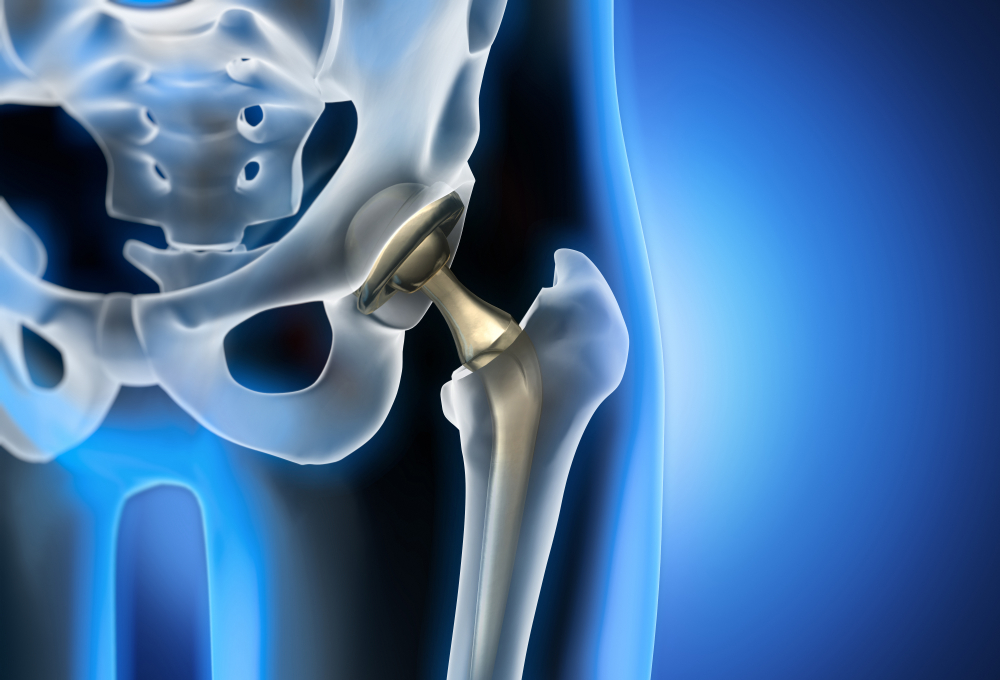
Depuy ASR XL Modular Acetabular hip replacement system was approved by the FDA using the 510(k) process in 2005. There were claims that Depuy knowingly kept the defective device on the market for years, putting company profits over the safety of consumers and patients. Depuy did not issue their voluntary recall until 2010.
Orthopedic surgeon Doctor Stephen Tower was one of many who suffered from cobalt poison after he was implanted with Depuy ASR Hip Replacement (metal on metal). After Dr. Tower had the product replaced he began to study the effects of the products in his very own patients. He believed the cobalt poisoning from the hip implant caused sudden decline in psychological health. Dr. Tower worried patients were being falsely diagnosed with Alzheimers or dementia, a permanent condition, versus cobalt poisoning, a reversible condition. Dr. Tower’s studies, however, did not generate much support with the FDA or Central Disease Control (CDC).
Manufacturers Stand Behind their Faulty Medical Devices
According to The NY Times, Depuy claimed they were phasing the implants out of the market due to slow in sales in 2009. Bayer, the Essure permanent birth control manufacturer, released a similar statement July 2018 claiming they would no longer sell Essure by the close of the year for the same reason. Unlike the Depuy hip replacement system, the FDA approved Essure by the more extensive PMA process, Footage from board meetings regarding Essure indicate clinical trials were short and had a low number of patients. It was approved despite multiple unanswered questions, including how the product would be removed if necessary.

“Plaintiffs allege personal injuries from the use of Essure, including hysterectomy, perforation, pain, bleeding, weight gain, nickel sensitivity, depression and unwanted pregnancy, and seek compensatory and punitive damages,” Bayer said in their Q1 report.
Trust your Instincts and Ask Questions
Naturally, when a doctor suggests a new treatment or surgery, the patient is likely to trust their doctor’s guidance. “The Bleeding Edge” strongly advises patients to get second opinions before deciding to go under the knife with a “new” procedure or agree to be implanted with “innovative” products.
If you have not already, we encourage you check out the The Bleeding Edge. It is available to stream on Netflix. It is a frightening, yet enlightening documentary. One thing is clear, the FDA needs drastic reform to their approval processes for medical devices and pharmaceutical drugs. We have learned over the years that in large corporations and Big Pharma will not self-police themselves. We hope that this expose will be a step in right direction to make America safer in the days ahead.
Other Medical Devices News
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
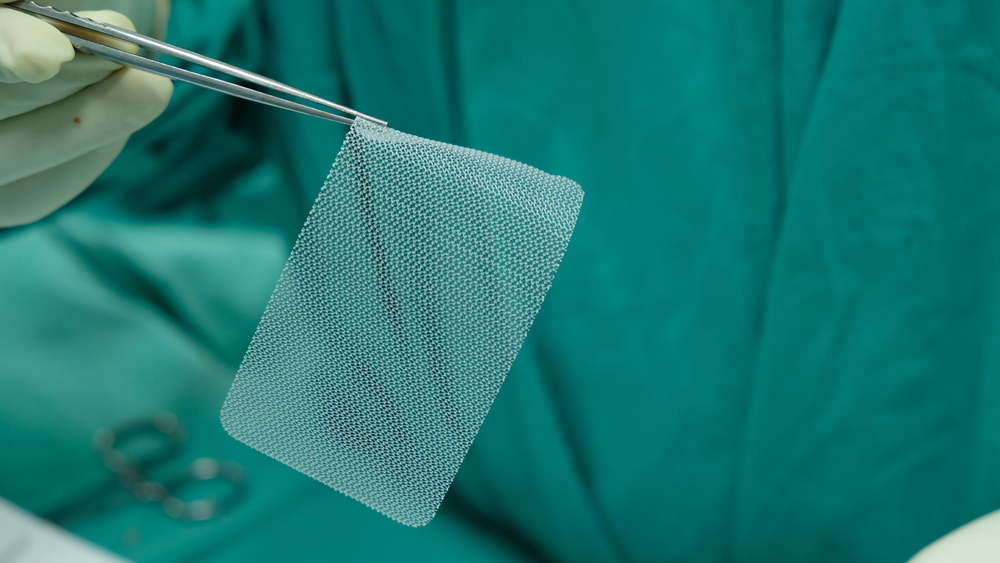
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
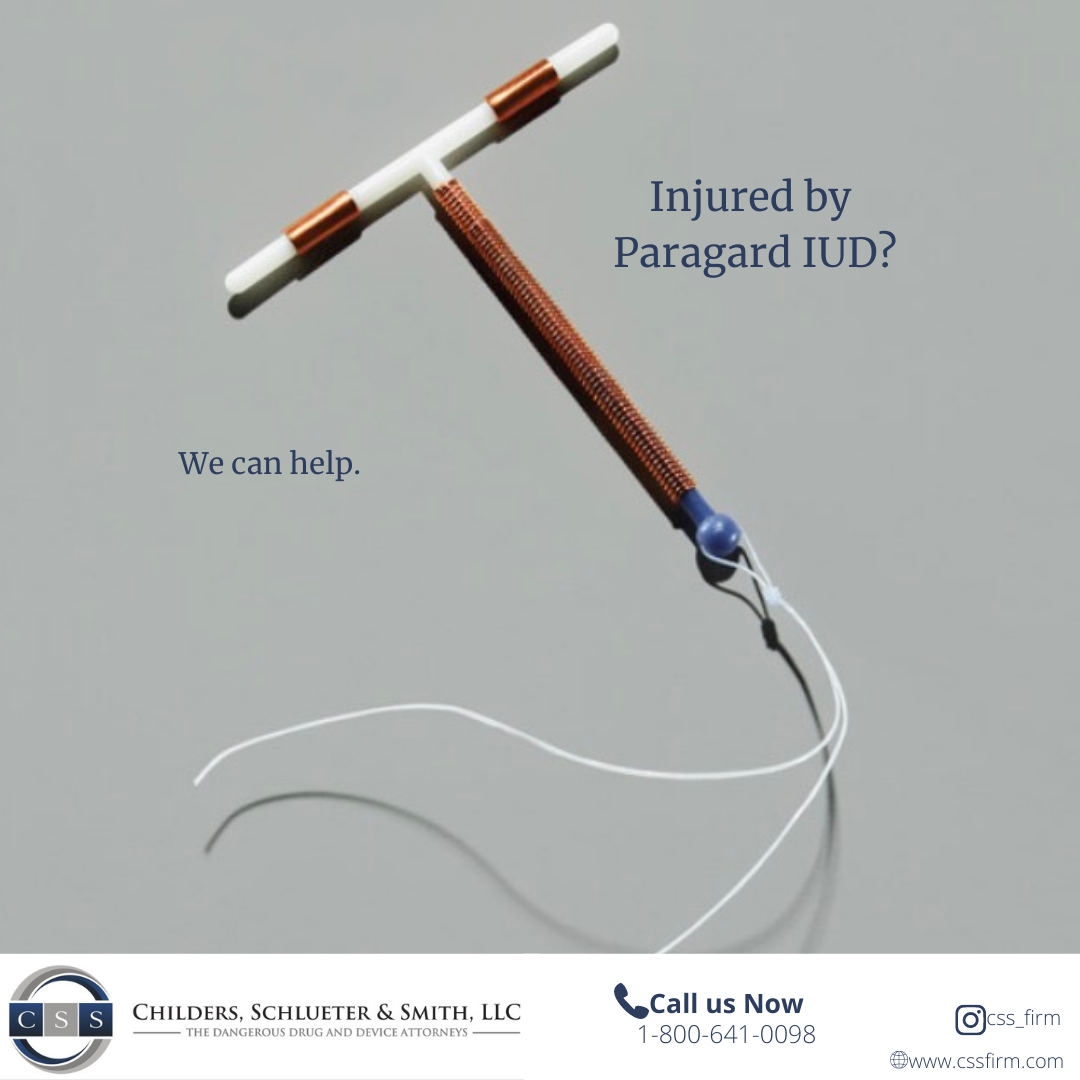
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.
A voluntary recall affects over 3.5 million Philips Respironics CPAP, BiPAP, and ventilator devices due to foam degradation risks, potentially endangering sleep apnea patients’ health and safety.
Philips Respironics has recalled certain CPAP, BiPAP, and ventilator devices due to degrading sound foam that may release harmful particles and chemicals, posing serious health risks to users.