Marketing Problems for DePuy
In its warning letter to DePuy Orthopaedics, Inc.’s president David Floyd on August 19, 2010, the FDA addressed concerns over the marketing materials connected to the TruMatch Personalized Solutions System and the Corail Hip System. The agency warned DePuy that some of the marketing materials it distributes are illegal under FDA rules, specifically mentioning marketing materials presented on DePuy’s website in the Products section.
The Johnson & Johnson division was given 15 days by the FDA to respond to the letter, but no response has been seen, other than the small public statement. It is notable that Corail marketing materials were quietly removed from DePuy’s Product section on its website, although the TruMatch materials remain.
Allegations of Providing Misleading Information
In the letter, the FDA alleges that DePuy provided misleading information to hasten the approval of the Corail hip implant. Errors were noted about information in both the product’s design and use. The approval process, legislated under 510(k), allows premarket approval of products but requires the product be functionally equivalent to another previously approved device. According to the FDA, DePuy’s brochure shows several assertions about the medical devices specifications and capabilities that were never approved by the FDA. In addition, the FDA alleges that DePuy never informed the agency that it planned on commercially distribute TruMatch.
Although DePuy responded with a statement, “We are reviewing the letter to understand the FDA’s concerns and will respond to their request for information,” the company also asserted that it did not believe they needed FDA clearance for the TruMatch system, a method of mapping the shape of an individual’s knee in order to create a knee replacement device that uniquely fits the individual.
The DePuy unit of Johnson & Johnson has faced many other issues regarding quality and safety on hip replacements. Soon after the FDA warning, DePuy recalled two more hip replacement products, creating a total of 10 recalls this year alone for parent company Johnson & Johnson.
Quality appears to be a major issue with the company in recent years. The McNeil Consumer Healthcare unit within the company has recalled several OTC medications since the latter part of 2009. In April, a recall was issued for over 136 million bottles of liquid children’s medicines because of excessive concentrations of the active ingredient and the presence of metal.
Contact Us
If you are having trouble with a defective product such as a hip replacement or knee replacement manufactured by DePuy Orthopedics, it is important you contact our office to find out what can be done about your failing implant. We can evaluate your case and help you decide if you should file a claim against the company for providing a defective medical device.
Other Medical Devices News
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
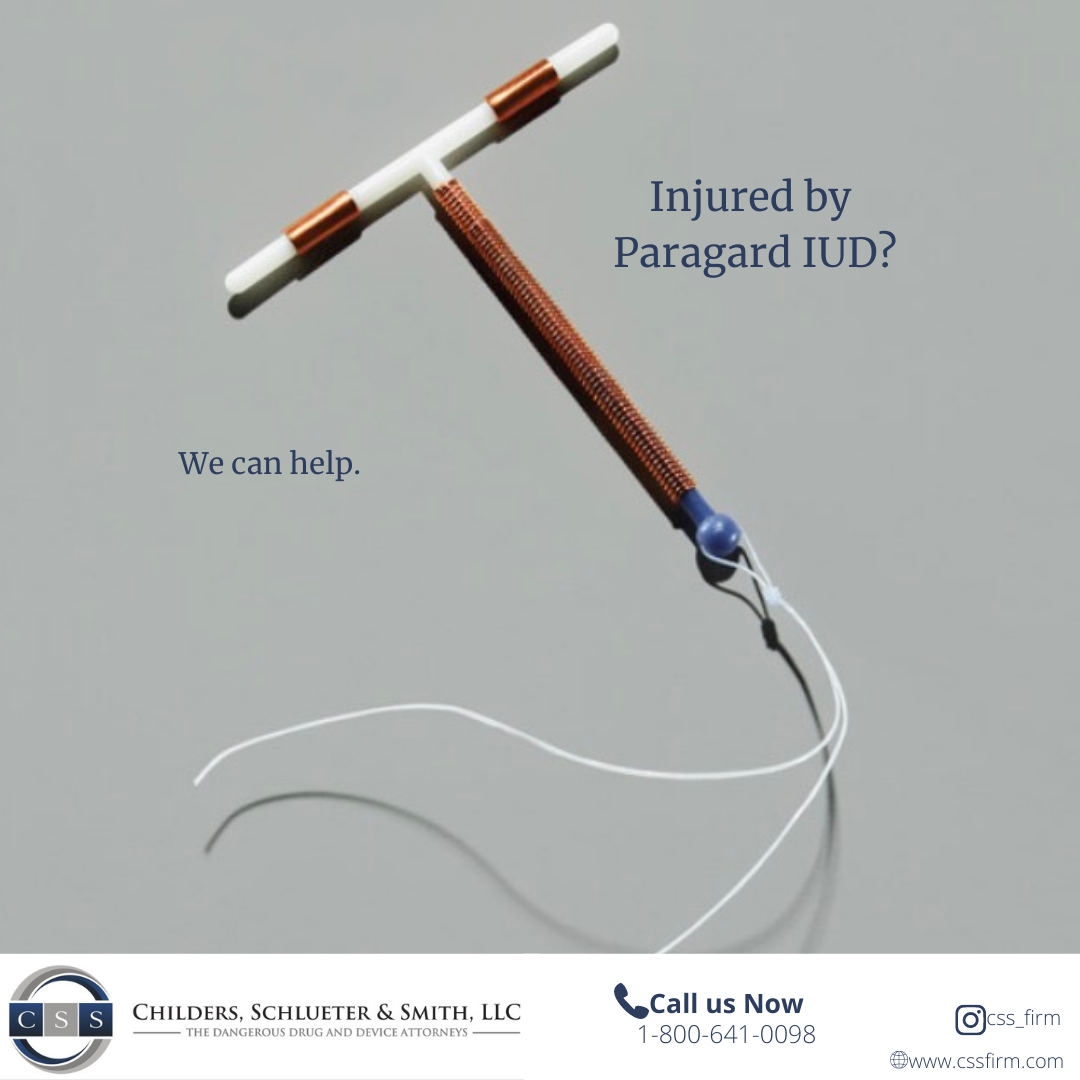
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.