Following on the heels of similar moves in Finland, the Netherlands, the United Kingdom, and Brazil, Canada has decided to withdraw the permanent birth control device Essure from the market within its country.
Following on the heels of similar moves in Finland, the Netherlands, the United Kingdom, and Brazil, Canada has decided to withdraw the permanent birth control device Essure from the market within its country.
According to a written statement provided to the Canadian press by Bayer, the recent decisions to drop Essure were made strictly for financial reasons, due to changes in patient demand, and the decision “is not a recall of the product from the market.” The device is the subject of thousands of lawsuits in Canada filed by women who allege that they were forced to undergo hysterectomies while in their 20s and 30s due to complications related to Essure.
Essure Permanent but Risky Method of Birth Control
Essure is a permanent birth control device that is not intended for removal, meaning women should only consider using it once their family is complete and they have decided not to have any more children.
The U.S. Food and Drug Administration (FDA) approved Essure in 2002. The device consists of a delivery system and two nickel-containing metal coils that are inserted through the vagina and implanted within each fallopian tube. The presence of the coils stimulates the growth of scar tissue, resulting in a tubal occlusion and sterilization of the woman.
Over the past several years, the FDA has been investigating a growing number of adverse events associated with Essure, leading the agency to convene a meeting of the Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee on September 24, 2015. At the meeting, expert opinions and patient experiences related to the risks and benefits of Essure were discussed.
On the basis of this meeting and the comments received, the FDA concluded that some patients are not receiving or comprehending information explaining the risks of benefits of permanent birth control implants like Essure, and as a result, the agency issued a boxed warning and decision guide for the device.
Other Medical Devices News
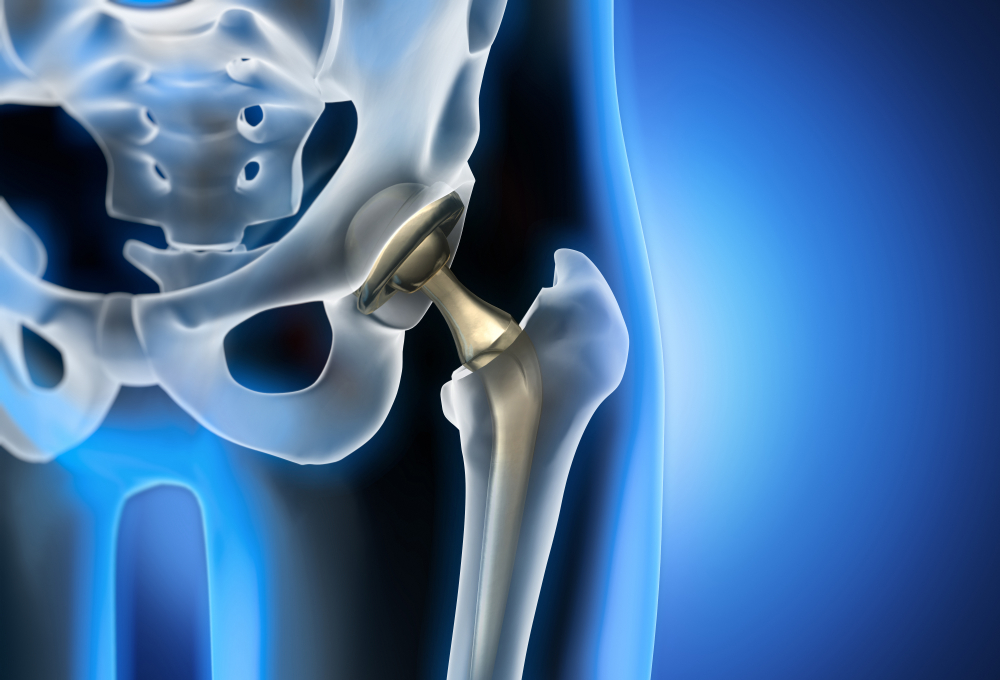
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
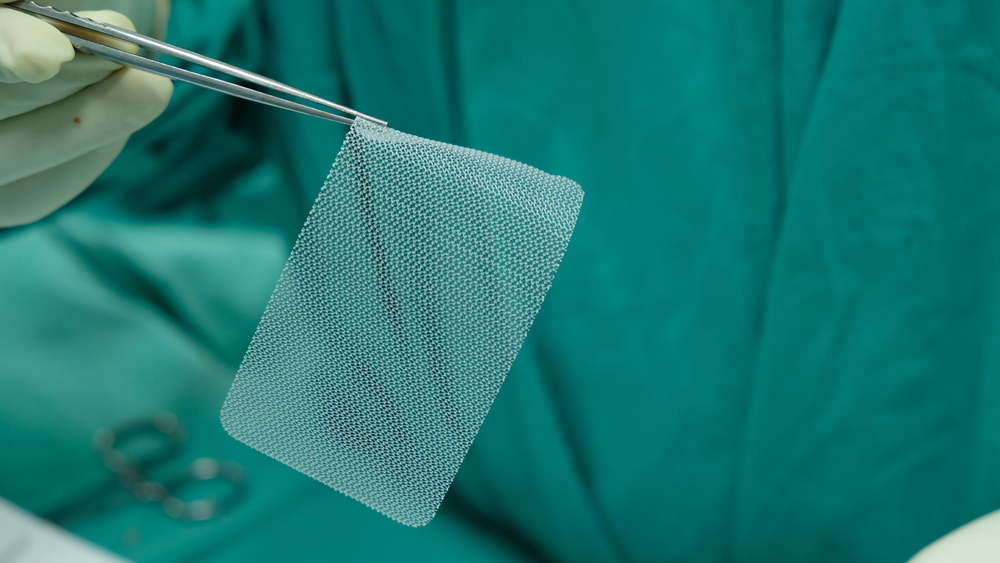
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
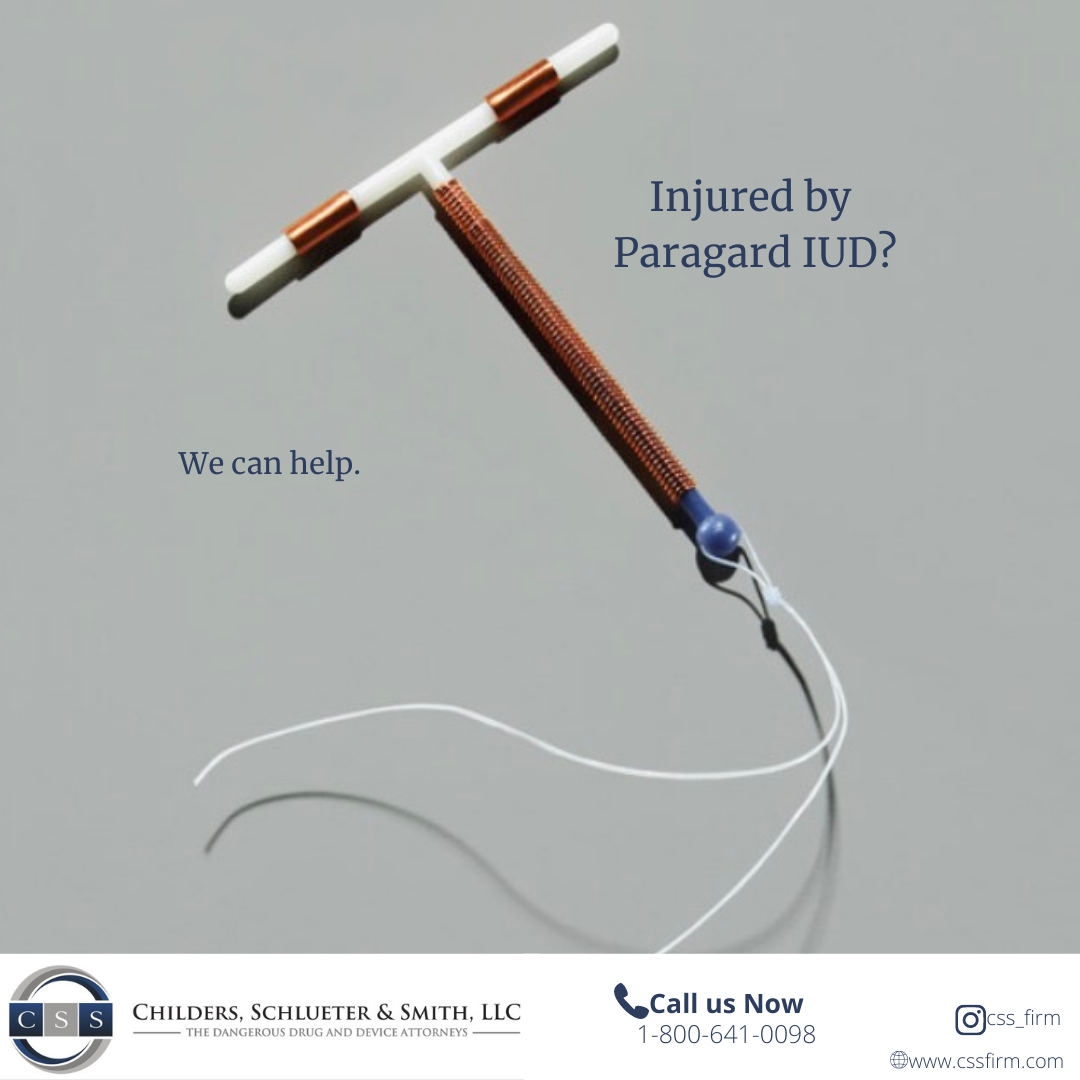
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.