After the surge of complaints reported on hip implant medical devices, there have been recent warnings of not becoming a victim of medical marketing.
After the surge of complaints reported on hip implant medical devices, there have been recent warnings of not becoming a victim of medical marketing.
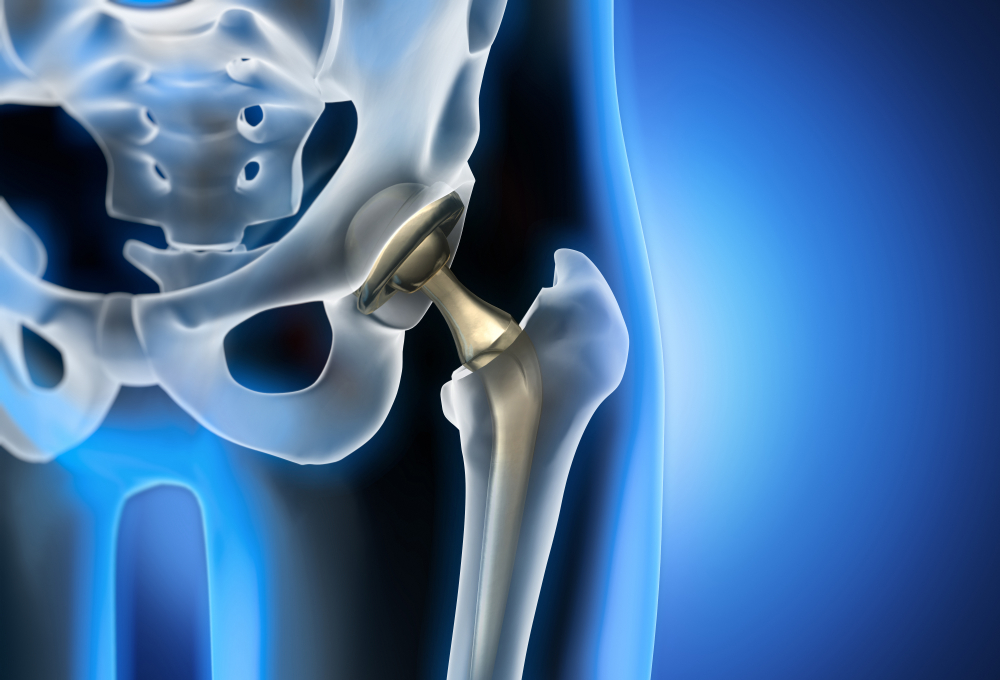
CNN recently published an article asking this question: “How likely is it that your doctor has a tie to a company that makes drugs or devices?” Dr. Robert Steinbrook wrote an article in August in the New England Journal of Medicine saying, “Most physicians in the United States have financial relationships with industry, ranging from the acceptance of meals to the receipt of large sums of money for consulting, speaking, or conducting research.” This is all coming to light after DePuy Orthopaedics recalled the ASR Hip Replacement System, which uses a metal-on-metal device.
There has been evidence that two physicians made more that eight million dollars each from DePuy Orthopaedics Inc. Many doctors do not think they will be influenced by these financial relationships but research has shown otherwise.
There have been thousands of complaints about the ASR Hip Replacement recalled medical device. The metal in the implant contains elements of cobalt and chromium, which have proven harmful and hazardous. Pieces of the metal from the medical device grind of the ASR hip implant that damages the surrounding muscles and tissues. The reported problems have been intense leg, groin, or hip pain, loose-fitting implants, fractures, friction transfer, and implant dislocations.
In the featured CNN article of not becoming a victim of medical marketing, they suggest a patient find out whether his doctor has financial ties to an industry. Experts say to look at the following hints: (1) look at pens and pamphlets; (2) ask questions about the devices: “If you’re considering getting an artificial knee or hip, you can check with the Association for Medical Ethics to see whether your doctor has a financial tie to Dupuy Orthopaedics or Zimmer Inc., Biomet Inc., Smith & Nephew Inc. or Stryker Orthopedics. All were required last year by the U.S. Department of Justice to disclose consulting agreements with physicians.”; (3) and ask questions about the drugs you will take long-term.
Other Medical Devices News
The FDA warns that Synovo Total Hip Systems, implanted after 2019, pose risks of failure and injury. Patients experiencing symptoms should consult our CSS lawyers to explore legal options for compensation.
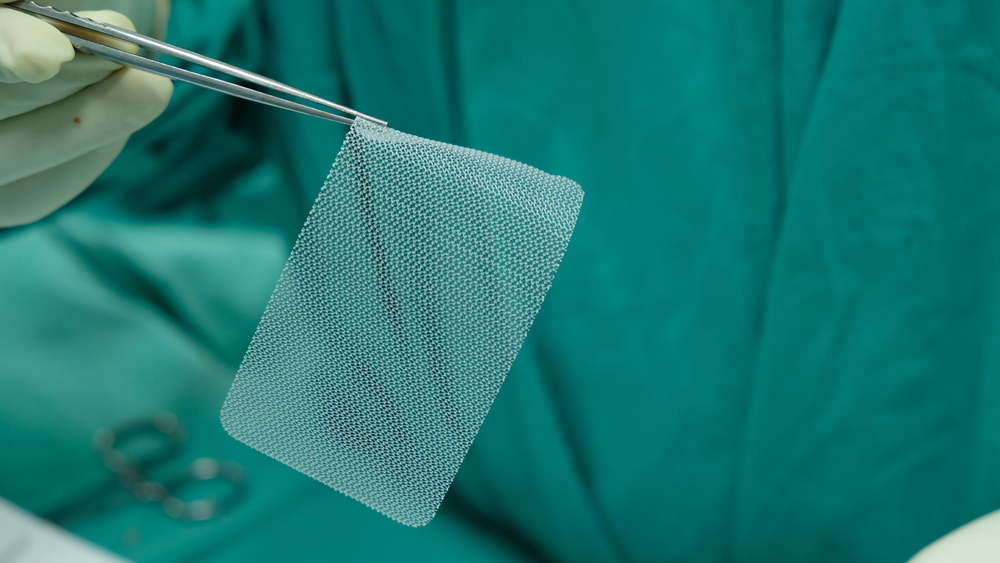
Thousands of hernia mesh products have been recalled, but qualifying for a claim requires proof that a defective hernia mesh implant caused your injury.
Damages from a defective hernia mesh implant vary based on each person’s injuries and circumstances. An experienced dangerous medical device attorney can help identify all the losses you may be entitled to recover.
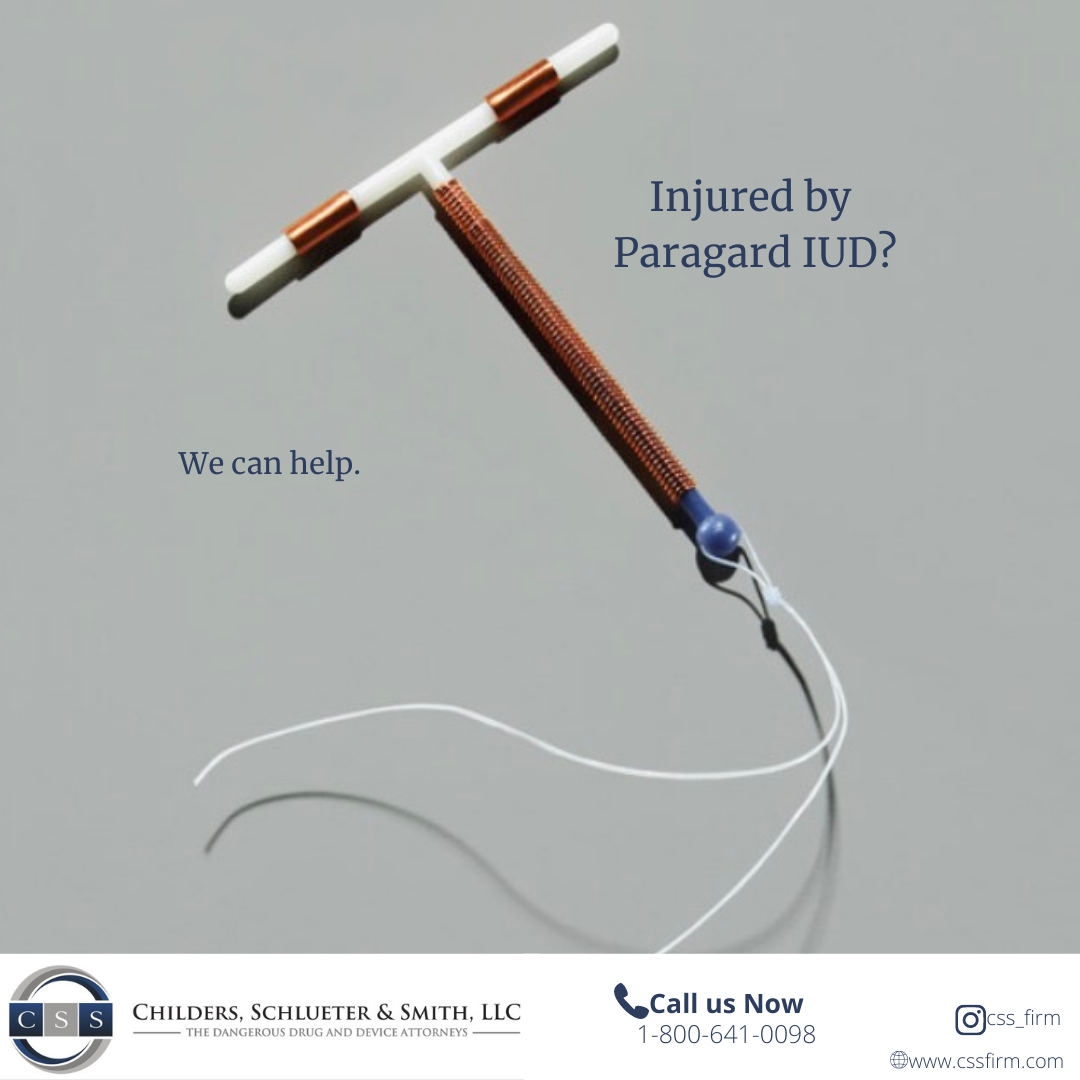
Women nationwide are filing lawsuits after suffering injuries from Paragard IUD breakage—alleging the manufacturer failed to warn of the device’s dangerous design defects.
A voluntary recall affects over 3.5 million Philips Respironics CPAP, BiPAP, and ventilator devices due to foam degradation risks, potentially endangering sleep apnea patients’ health and safety.
Philips Respironics has recalled certain CPAP, BiPAP, and ventilator devices due to degrading sound foam that may release harmful particles and chemicals, posing serious health risks to users.