CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only.
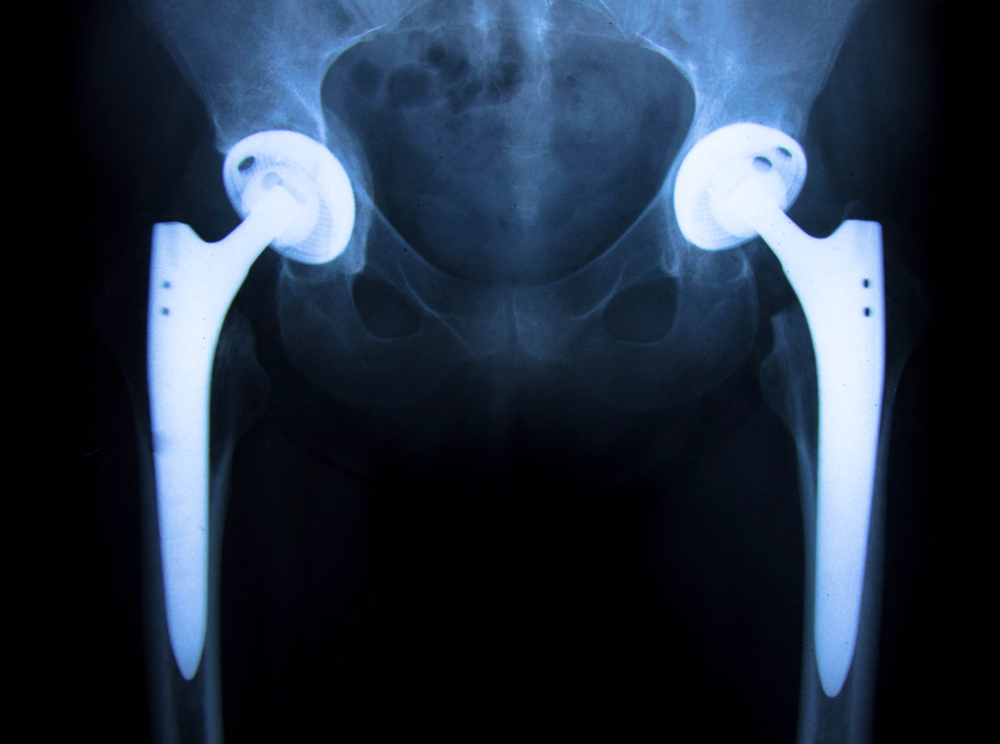
Stryker is facing yet another serious problem with their total hip replacement medical devices, this time related to the LFIT-V40 chrome/cobalt heads, which have been reported to potentially cause metallosis or catastrophic dissociation requiring revision surgery. The company previously survived a major recall of its Stryker Rejuvenate and ABGII modular stems in 2012.
Metallosis
In metal-on-metal hip implants, both the head and the liner (or acetabular shell) are made of similar metals, typically cobalt, chromium, molybdenum, and titanium. Friction, or wear, can cause a corrosive process to occur, which may result in severe pain and the need for revision surgery. This process can also lead to elevated metal levels, resulting in a condition known as metallosis.
Metallosis is a relatively rare condition that occurs when metallic debris builds up in the soft tissues of the body. Although metallosis has been observed in various individuals who have undergone hip replacement surgery, patients who have received fully metal implants are most likely to suffer from the condition.
Catastrophic Dissociation
The effects of catastrophic dissociation can be much more serious than a typical metal corrosion case. With catastrophic dissociation, the neck of the stem is weakened to the point of breaking, and if corrosion has eaten away at the neck and caused a break at the head-neck junction, the patient will need a much more invasive revision surgery with complete removal of a well in-grown stem.
Removal of a well in-grown stem is a very serious operation that is susceptible to many complications, and a surgeon typically won’t even try to perform this operation, instead referring the patient to a specialist.
Stryker Hip Lawsuits
Stryker lawsuits have been consolidated into multi county litigation (MCL) in the Superior Court in Bergen County, New Jersey, and into multidistrict litigation (MDL) in the U.S. District court of Minnesota. Atlanta law firm Childers, Schlueter & Smith, LLC has filed more than 25 additional Stryker hip cases in the last six months on behalf of injured clients from all over the U.S.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.