Do you have a defective knee that needs to be replaced or revised? Do you know why it failed? Do you know what device the surgeon is suggesting to do the repair with? Are you aware of what the success or failure rates of the revision components? Our firm has reviewed thousands of examples of prosthetic failures and can help you get answers. In fact we have a focus now on the DePuy Synthes Attune Knee Replacement system.
Do you have a defective knee that needs to be replaced or revised? Do you know why it failed? Do you know what device the surgeon is suggesting to do the repair with? Are you aware of what the success or failure rates of the revision components? Our firm has reviewed thousands of examples of prosthetic failures and can help you get answers. In fact we have a focus now on the DePuy Synthes Attune Knee Replacement system.
Revision surgery for the replacement of an initial index implanted knee due to premature failure is unfortunately all too common. When the failure occurs where there is not an antecedent fall, motor vehicle incident or other trauma related event, many times it’s the design or metallurgy of the device that is the problem. Often times there is systemic inflammation from various materials including titanium (TI), Cobalt- Chrome (CO-CR) various polymers, ceramics that result in frictional wear, metallosis or galvanic corrosion that is gradual or leads to a sudden mechanical failure.
The patient rarely picks the component or the manufacturer. How do you know what is going to be used before it is implanted? Simple, Just ask.
More than 150 different knee replacement devices are available from a variety of manufacturers, including:
• Biomet, Inc.
• ConforMIS
• DePuy Synthes
• Exatech, Inc.
• Smith & Nephew
• Stryker
• Wright Medical Group N.V.
• Zimmer Inc.
To research a failed implant or to find out if there are problems with the device you are being revised to, go to the FDA database for information on whether the products have a reported history of failing and why. To find physicians in your area by zip code, go to the websites of the American Association of Hip and Knee Surgeons (AAHKS) or the American Academy of Orthopaedic Surgeons (AAOS).
Knee Replacement Failure
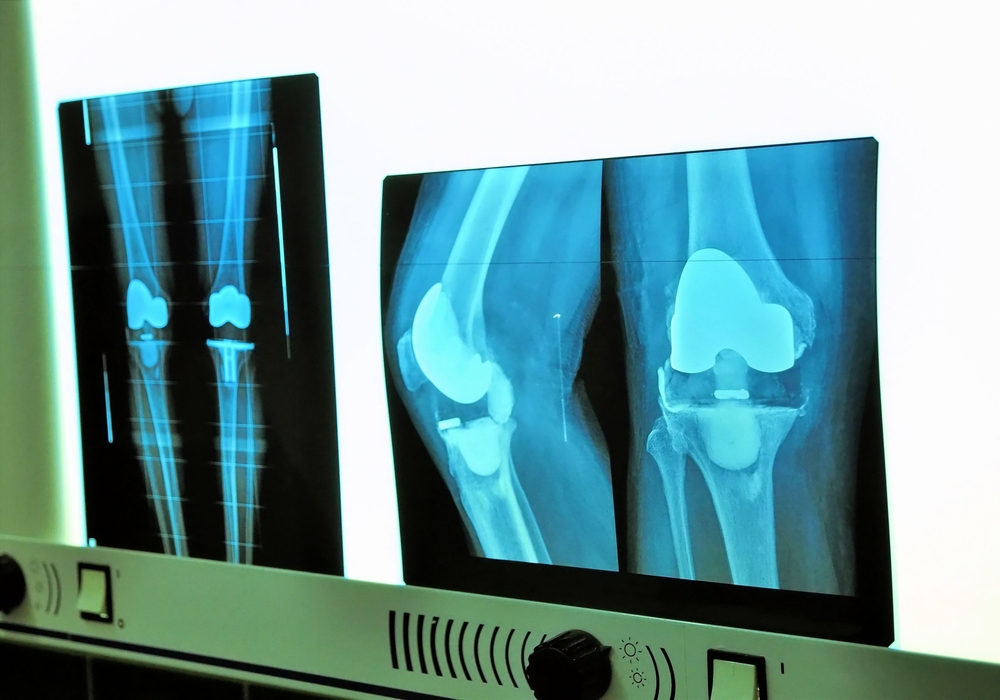
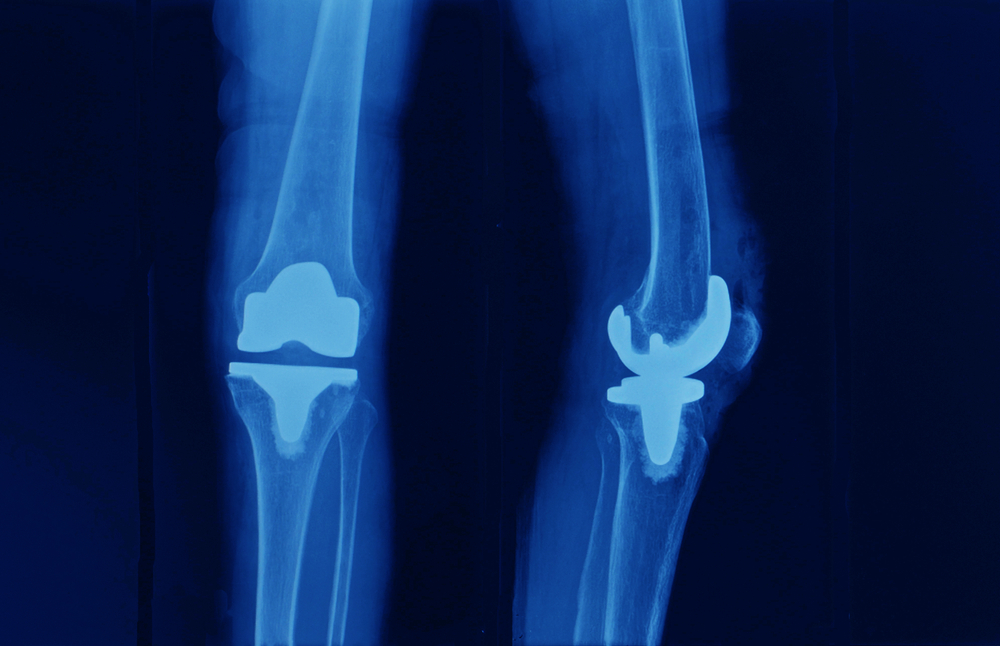
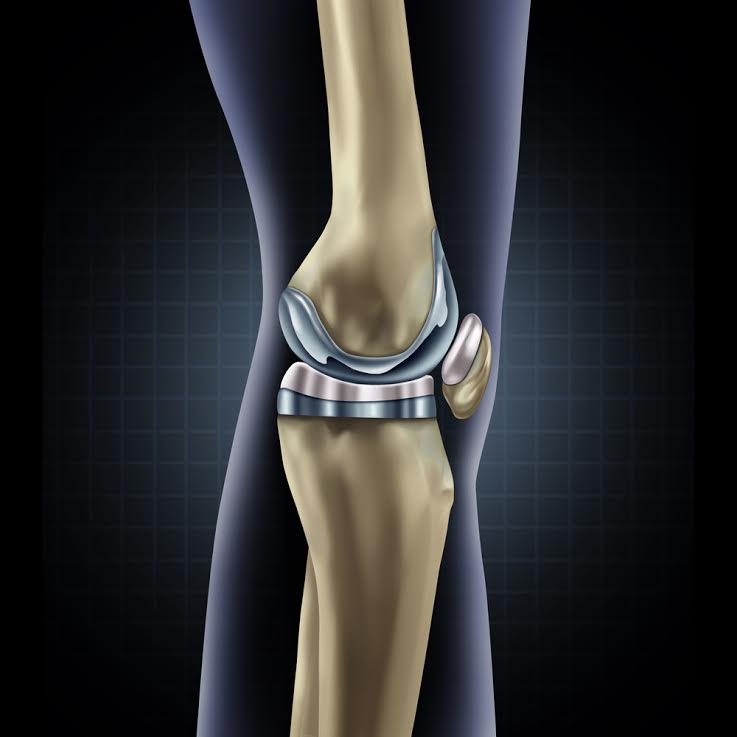
A knee replacement system consists of two main components – a tibial trey and a femoral implanted component. The weight of the patient is transferred between these two components, allowing for activity and motion. The joint is typically constructed with a surface of plastic or polyethylene construct, sometimes in a single piece or in a modular construct involving multiple, interchangeable pieces.
When these devices fail because of component loosening or premature wear, this failure can necessitate an additional surgical procedure. Frequently, the medical expenses, loss of work and need for rehabilitation associated with this procedure exceed $50,000 to $100,000, not accounting for the individual patient harm that may be substantial.
Please Note: CSS Firm is no longer accepting or investigating new knee implant claims at this time. This article is for educational purposes only.
Other Knee Implant Lawsuits News
Tibial debonding in knee replacements can cause implant loosening and require revision surgery. Learn how this complication occurs, its risks, and potential legal considerations for affected patients.
Aseptic loosening, the leading cause of knee replacement failure, can result in pain, reduced mobility, and revision surgery. Learn the causes, risks, and potential treatment options for affected patients.
Knee replacement base plate loosening can cause pain, stiffness, and instability, often requiring revision surgery. Learn how design, sizing, and manufacturer issues may impact implant longevity and patient outcomes.
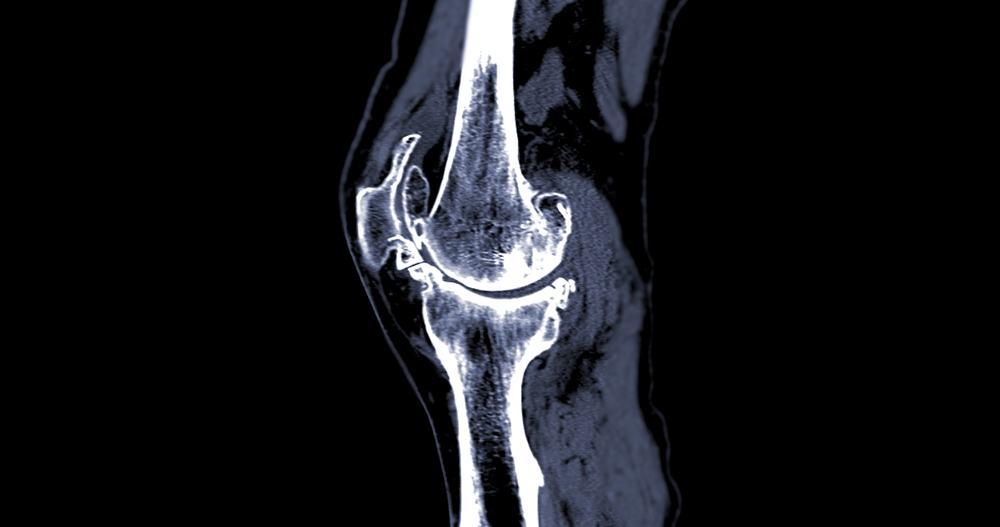
A bone scan can help determine the condition of a knee joint or implant before replacement or revision surgery, offering surgeons critical diagnostic insights not always visible through standard imaging.
The first lawsuit over premature failure of the DePuy Attune knee implant has been filed, highlighting concerns about tibial base plate loosening and potential widespread complications among patients.
Knee replacement surgery can relieve pain and restore mobility, but some implants, including the DePuy Attune Knee System, have been linked to premature failure and complications requiring revision surgery.