On January 3, 2013, Stryker announced that it had hired Broadspire, a third party claims administrator and subsidiary of Crawford & Company, to handle the Stryker Rejuvenate and ABG II Modular-Neck Stem Hip Implant recall lawsuits and related injury claims. Broadspire is the same entity that has been retained by DePuy Orthopedics in the DePuy ASR recall that was announced in 2010. We would encourage consumers to read up on them at their website: http://www.choosebroadspire.com/.
The Parent company, Crawford and Company, claims to be the world’s largest insurance and claims adjuster. Broadspire will seek to gain access to the medical records of patients affected by Stryker’s recent hip implant recall through medical authorizations being provided by the implanting surgeon’s office, or directly by Broadspire itself.
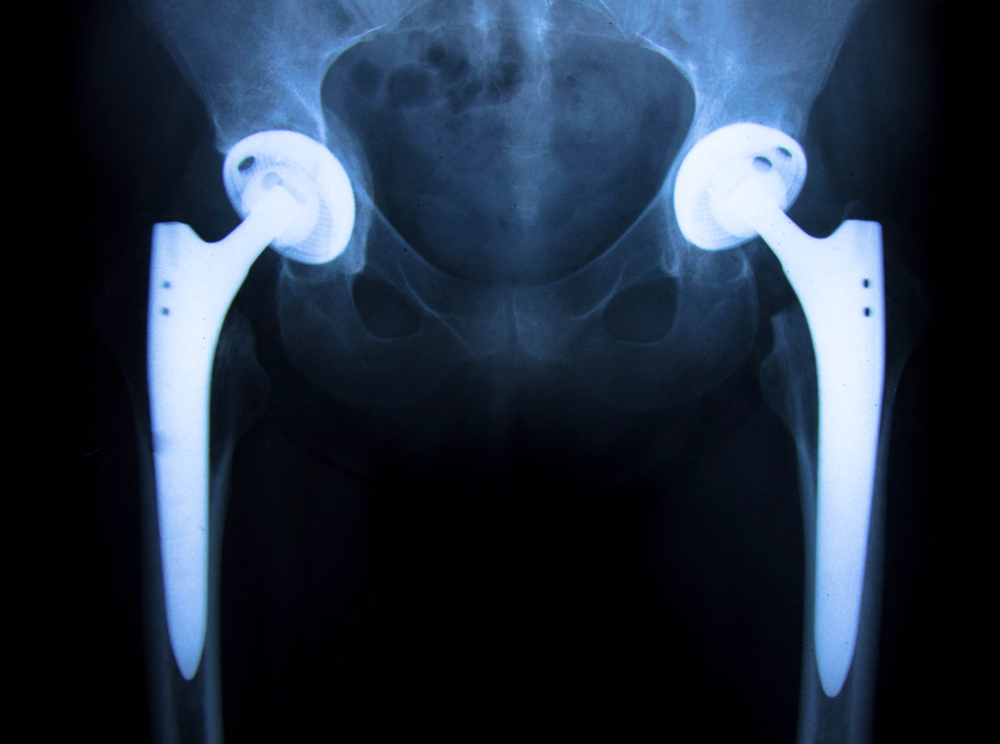
In June, 2012, Stryker recalled two versions of the company’s artificial hip implants: Stryker Rejuvenate and Stryker ABG II Modular-Neck Stem.
According to reports, thousands of recall letters to hip replacement recall victims have been sent notifying patients directly from doctor’s offices. These letters ask patients to sign medical authorization forms and provide other personal forms of information. The forms would release detailed information contained in the patients’ medical records to Stryker and third party contractors. It also allows Stryker to have unfettered access to patients’ medical providers and further allows them the ability to inquire into unrelated medical conditions and issues without notice to the patient.
The reality of this situation is that if a patient signs this release form without other safe guards being in place when doing so, they effectively waive their federally protected rights to medical privacy and the information released to Stryker can be used as evidence in court against them as their claim progresses or in the alternative, of cost containment of medical billing review. The majority of consumers have done little in the way of researching the purpose of Broadspire and their role of representing Stryker. This potential for abuse of utilization of medical information gathered, based on the conflicting role of Broadspire interest, is all too familiar. We have been dealing with these issues and Broadspire for years now and, therefore, know how to navigate these important issues for our clients. Because many doctors and their offices require these forms to be completed and submitted to Broadspire for continued care and treatment, it is important to talk to an experienced group of hip implant attorneys to better understand the best way to do this.
Before signing anything, you need to contact the Stryker Rejuvenate Recall Attorneys at Childers, Schlueter, & Smith, LLC for a free consultation and review. Childers, Schlueter & Smith, LLC continues to investigate and review the claims of patients nationwide who received a Stryker hip implant device. If you, or a loved one, has received the Stryker Rejuvenate and/or Stryker ABG II Modular-Neck Stem and have experienced some type of hip pain, elevated blood ion levels, developed metallosis, required an additional hip surgery, or any failure with your hip replacement device, you may want to contact CSS Firm for a free case evaluation and consultation. Our experienced team of hip implant lawyers will work closely with you and see to it that you get the medical treatment you need and that your legal rights are fully protected.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.