More than 570 federally-filed Stryker hip lawsuits have been filed in a multi-district litigation (MDL) now underway in the U.S. District Court, District of Minnesota. According to documents filed March 14, 2014, the Court anticipates selecting 3-5 bellwether cases for trial sometime during the Summer of 2015.
The Minnesota District Court will continue to reach out to New Jersey and Florida state court judges to explore the potential of a coordinated settlement of all the state and federal cases involving Rejuvenate and ABG II. At the present time, nearly 800 Stryker hip lawsuits have been filed in Bergen County Superior Court in New Jersey, and 13 such cases were consolidated in Florida on October 3, 2013.
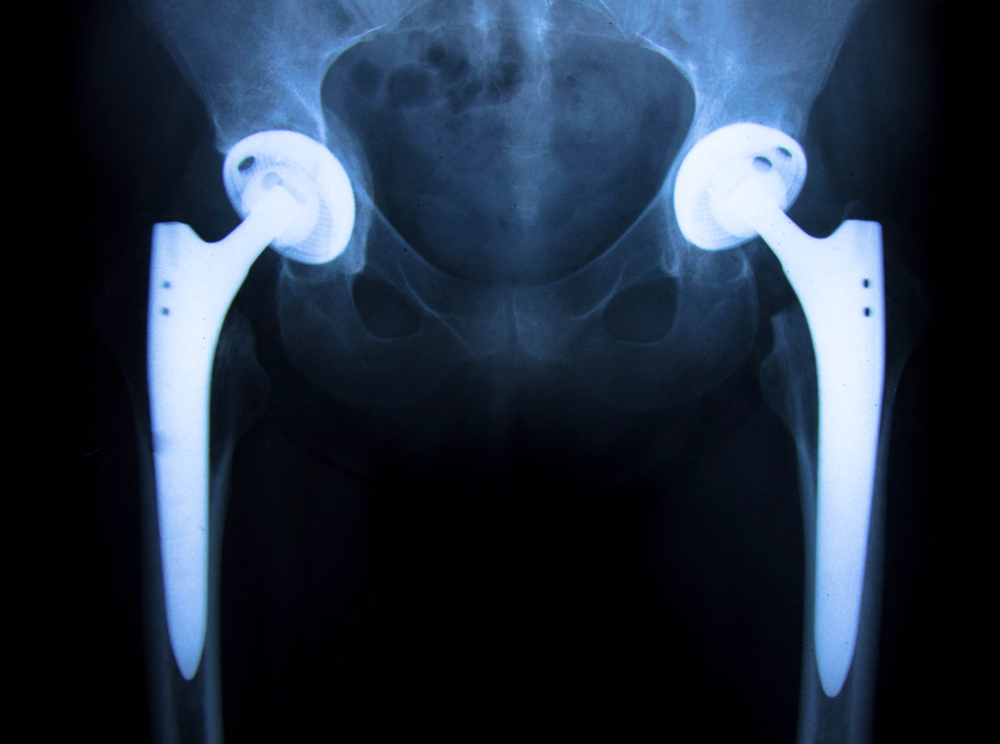
All of the lawsuits involve similar claims regarding the tendency of the Rejuvenate and ABG II hip stems to fret and corrode, causing the recipients to experience osteolysis, tissue necrosis, and other complications that require replacement of the device. Revision surgery is generally more difficult and takes longer to perform than the initial hip replacement, according to the Orthopaedic Research Institute.
Stryker recalled its Rejuvenate and ABG II hip stems in July 2012 after the company determined that the metal hip stems could fret and corrode at the modular-neck junction. The company has recommended that all Rejuvenate and ABG II recipients undergo blood and imaging tests to ensure that their hip stems are functioning properly, even if they have not experienced any symptoms of a failing hip implant, which include thigh pain that radiates to the knee, particularly while walking or going from a sitting to a standing position.
Although a typical hip replacement is expected to last between 15 and 20 years, the defects with the Stryker Rejuvenate and ABG II hip implants were detected in less than two years. Other metal on metal hip implant manufacturers have had similar issues, including DePuy maker Johnson & Johnson, who recently made offers to settle lawsuits involving nearly 8,000 patients for more than $4 billion.
The Stryker Hip Implant Lawyers of Childers, Schlueter & Smith continue to review and file Stryker hip implant cases. If you have questions about your rights, contact us today for a free evaluation of you potential Stryker hip implant lawsuit.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.