Failed Stryker hip implants could cause metal poisoning, tissue and bone damage, necrosis, osteolysis, and severe pain. Thousands of people have had to undergo revision surgery to replace these devices after suffering from a range of serious, painful, and life-limiting symptoms. Product defects have led to years of legal troubles for the manufacturer.
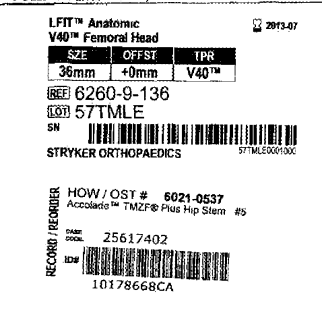
Stryker hip implant lawsuits have involved clams from the modular stems: Rejuvenate and ABG II. Also, with cobalt chrome head known as the LFIT V40 that fits monolithic stems that include the Accolade TMZF, Citation TMZF, and Meridian TMZF. Only the Rejuvenate and ABG II stem have been recalled, as well as certain sizes of the cobalt chrome v40 femoral heads of size 36mm and greater. Both of these failures have the tendency to have galvanic corrosion at the junction interface of the modular stem as well as with the trunnion when you have a cobalt chrome femoral v40 head mated on the titanium alloy stem. The older versions of the TMZF alloy stems have been associated with corrosion when mated with a cobalt chrome head. A similar head made of ceramic, when used, does not have these risks. The manufacturer claims regarding the v40 cobalt chrome head recall that it’s related to manufacturing tolerances of devices manufactured prior to 2011 of sizes 36 mm and greater. Nonetheless, many reports are seen in smaller sizes, with similar failures and claims regarding devices well after 2011.
Defective device lawsuits against Stryker have alleged they were negligent in several ways, including misrepresentation of safety, defective design, and failure to adequately test devices. They further claim that Stryker knew or should have known the design was defective and, ultimately, that they failed to create, market, and sell safe hip implants.
Several models and sizes of Stryker hip implants have been recalled since 2012. The health effects of recalled products are extensive, and it’s important to know that those affected by defective hip implants have medical and legal options.
Through our experience handling Stryker hip implant lawsuits, our attorneys have put together the top 10 things all Stryker hip implant patients need to know. If you’ve suffered due to a defective hip replacement and have further questions or want to discuss whether you have a case, contact our firm for a free case consultation.
1. When were Stryker hip implants recalled?
The first recall of Stryker hip implants involved two of the company’s products: Rejuvenate and ABG II modular-neck stems. The recall was announced in June 2012.
Years later, on November 9, 2016, the FDA announced a recall of Stryker LFIT V40 Femoral Heads (metal Cobalt Chrome heads) manufactured as early as 2006. The LFIT V40 recall expanded in 2018 to include catalog numbers beginning “6260 -9.”
2. Why were Stryker metal heads and modular-neck stems recalled?
Depending on the model and components used, there are numerous reported dangers that led to the recall of certain Stryker hip implants. These include metallosis, bone damage and breaks, serious infections, and more, all of which can be extremely painful.
Some of the most common ways a hip replacement fails are:
- Disassociation (detachment) of femoral head from hip stem
- Gross Trunnion failure (significant damage to the trunnion affecting proper kinematic motion)
- Fracture of the stem
- Corrosion of the stem
- Excessive metallic debris deposited in the surrounding tissue in the hip capsule
The Rejuvenate and ABG II modular-neck stems were recalled because they may corrode and release metal ions of cobalt and chromium into the body, affecting surrounding tissue and bone over time.
There are other complications caused by corrosion at the connection between the head and stem in Stryker V40 LFIT hip implants. Experts that our defective products lawyers have consulted with tell us the LFIT V40 femoral head is made of cobalt and chrome, which requires monitoring when matched with titanium-based stems such as the common Accolade TMZF stem. This mixed metal combination of titanium with cobalt and chromium results in wear and/or corrosion post-implant, resulting in serious health effects for the patient.
3. What are the signs or symptoms of a failing hip implant?
The most common signs and symptoms of a failing hip implant include pain around the hip, difficulty walking, instability, and swelling from inflammation. If a head-neck dissociation occurs, it’s extremely painful. Your physician should evaluate you to determine if you are at a greater risk of injury or complication.
The inflammatory response from the metal in Stryker hip implants may occur with little or no symptoms until there is harm to the surrounding tissue and loss of bone. An early X-ray is a decent way to examine the bone and device placement, but an MRI often provides more specific information. Without an MRI, patients may not recognize the harm occurring until they develop symptoms. (Aspiration from the joint or blood work to measure the metal ion levels is a telling sign of medical complications). Your orthopedic surgeon can help you with these matters. Call their office to discuss your plan of care and treatment if you have not done so recently.
4. Why is the level of cobalt important, and what is the FDA saying about monitoring?
Cobalt is one of the many metals found naturally in the body, but as is common with most metals, levels in excess can become toxic and lead to many harmful and potentially harmful side effects. Cobalt poisoning has caused cardiomyopathy, hypothyroidism, and neurological damage, as well as impaired sense of sight and hearing.
The FDA has identified additional concerns about metal-on-metal hip implants beyond the general risks of all hip replacements. Metal particles may be released into the bloodstream because metal components slide against each other while patients walk or run, and through other metal parts of the implant. It can damage the bone and tissue around the joint and implant, causing pain and even device failure, and lead to revision surgery earlier than normal.
As far as the recalled Stryker hip implant devices go, excess amounts of cobalt in the bloodstream lead to inflammation, which can seriously damage surrounding tissue and bone. If a doctor does not independently research studies regarding toxic values of cobalt, patients will likely not be informed if the level present in their blood is actually elevated or even toxic. This subjects them to prolonged issues and injuries from hip implants.
5. Is additional surgery needed for recalled Stryker hip implants?
Your doctor can only answer whether or not additional surgery is needed because you have a recalled Stryker hip implant. They’ll evaluate the risks and benefits, including the potential harm of removing and replacing the device altogether.
Many factors, such as age, health, and weight, must be carefully considered. Make an appointment with your orthopedic surgeon to discuss the potential problems associated with Stryker hip implants and other avenues of treatment to see what is best for you and your family. You will often find that surgeons with firsthand experience with the harms of recalled Stryker hip implants may not be the same ones who put in the device.
A second opinion is also worth considering. Be sure to ask what components would be used to replace a Stryker device and if the physician is limited by contract or experience to a certain manufacturer—experience related to these modular components matters.
6. What are the risks of hip replacement revision surgery?
There are risks to having any type of surgery, but knowing more about the procedure and how to deal with it is key. This is an excruciating and complicated procedure. A second hip surgery is usually longer and more intense than the first, and not all patients are candidates for revision surgery. Only a trained medical doctor can make this determination with you after conducting the requisite blood tests, x-rays, MRIs, and other medical examinations.
Unfortunately, thousands of patients with recalled Stryker hip implants had no choice but to have revision hip replacement surgery due to the serious health effects and risks of a defective device. Many patients who thought they’d never get another hip replacement in their lifetime ended up requiring revision surgery just a few years after the first one. They had to endure another long recovery and faced increased risks during the complex surgery.
7. Who will pay for my medical expenses for a failed Stryker hip implant if I am uninsured or have out-of-pocket costs?
Stryker hired Broadspire on or about January 3, 2013. According to reports, Broadspire is a division of Crawford and Company that is touted as the world’s largest insurance and claims adjuster. They’re funded by Stryker to gain access to the medical records of patients affected by the company’s recall of the Rejuvenate/ABG2 modular stems through medical authorizations.
There can be a benefit to the uninsured patient seeking reimbursement for medical expenses or gaining approval to have expensive procedures paid for by Stryker. However, keep in mind that making a claim for out-of-pocket expenses or medical bills not paid for by insurance is all that Broadspire is really doing based on our findings. Before seeking coverage through the company, get legal advice from a defective products attorney as soon as possible.
If you don’t file a Stryker hip lawsuit before the expiration of the statute of limitations, you will not be able to pursue compensation for the harm you’ve experienced or may experience in the future. These include but are not limited to pain and suffering from a failed Stryker hip implant.
8. What responsibility does my physician have?
One common question many of our firm’s clients have is what responsibility, if any, the doctors and/or orthopedic surgeons who implanted these devices have in this Stryker hip implant recall. Our firm’s research and findings are clear: Doctors have no responsibility or liability in the cases we have seen and reviewed to date. Here’s why.
Stryker made and marketed the device to your surgeon, and they failed to provide information prior to implantation of the increasing failure rates of Rejuvenate /ABG II Modular-Neck Stems and LFIT v40 metal heads. Had your surgeon known of the problems created by these products, they likely wouldn’t have used the implants. Given these facts, we see recalled Stryker hip replacements as a product liability issue, not one of medical malpractice.
9. What types of damages could a Stryker hip implant lawsuit seek compensation for?
A jury can consider the following list of non-economic damages for a Stryker LFIT V40 Femoral Head, Rejuvenate, or ABG II Modular-Neck Stem hip replacement lawsuit:
- Lost wages or loss of earning capacity.
- Effects on mental and physical well-being.
- Duration and extent of the injury/harm.
- Mental anguish and pain.
- Amount of past or future medical expenses.
- Extent of any disfigurement or scarring.
Thousands of Stryker hip implant lawsuits became part of MDL in 2013 and again in 2018. Settlements were reached with consideration of the damages listed above, and in general, most plaintiffs accepted. However, many chose to pursue their own lawsuit, and some are still pending today.
10. Should I surrender my defective hip implant to Stryker?
The position of our Stryker hip implant attorneys is that you should keep the implant hardware with an approved medical device retrieval company per an agreed protocol. This is a very important decision because the removed hardware will become critical evidence in your lawsuit. It is your hardware because you or your insurance company paid for it, so don’t let anyone tell you otherwise.
We have retained a company that works directly with the hospital and surgeon to ensure the device is preserved for any subsequent testing and/or failure analysis. If you have questions about the retention of your implanted hardware, give us a call, and we will be happy to help.
Hire a Stryker Hip Implant Attorney
Along with the medical costs from eventual revision surgery, a failed hip replacement can cause a lot of other hardships; it may affect the patient’s life and lifestyle and overall physical, financial, and emotional health. Too many patients with defective Stryker hip replacements have struggled through extensive rehabilitation therapy to get back to a more active life – only to have their daily activities slowly restricted again when their Stryker device began to cause problems. For this and other reasons, our hip implant clients are represented on an individual, one-on-one basis. (We are not representing plaintiffs in a class action, nor do we believe it would be in your best interest to be part of one).
Our tenacious legal team is committed to getting the best results for those harmed by Stryker hip implants. While Stryker claims it will cover reasonable and customary costs of monitoring and treatment of certain components, their intentions may not be so transparent. They’ve requested that patients submit all their bills to private health insurance like Medicare and employer health insurance plans, offering only to pay out-of-pocket co-pays and deductibles. This is an excellent deal for Stryker, but it is not justice for the patient or your health insurance carriers, who are, in essence, paying for the company’s mistakes.
At Childers, Schlueter & Smith, we’ve been protecting the rights of people who have suffered because of defective hip replacements and gotten them the compensation they deserve for the hardships and harm they’ve suffered. Given reports of additional lots and models of Stryker hip implants failing and the need to file a civil claim within the statute of limitations, it’s essential to contact our experienced team to discuss your case. Contact us online or call us at 1-404-419-9500 for a free consultation.
CSS Firm is no longer accepting new Stryker LFIT V40 Hip Implant Claims. Any information on this page or website is for educational purposes only on that topic and device.
Other Stryker Hip Implants News
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.
Stryker’s track record includes multiple recalls and lawsuits involving defective hip implants—and recent reports suggest continued failures in other device components, raising serious safety concerns.