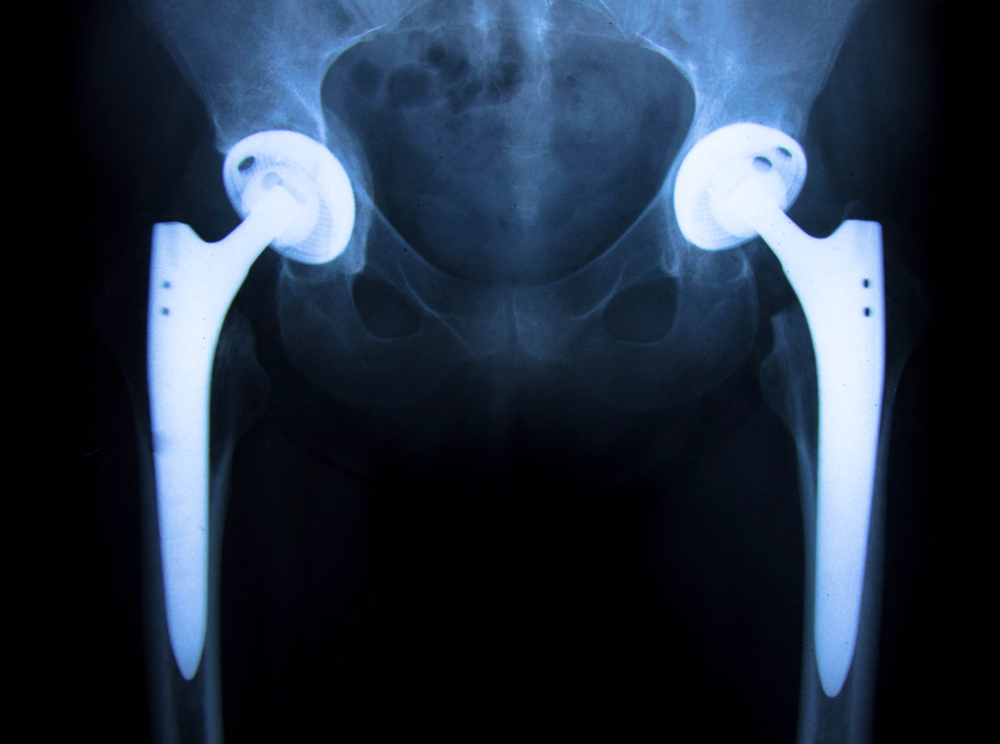
Our personal injury lawyers are still investigating claims on behalf of anyone who has had revision surgery due to complications from the recalled Stryker Rejuvenate or ABG II modular-neck stem implant. In July of 2012, the Stryker Corporation issued a voluntary recall of the Rejuvenate and ABG II modular-neck stem hip implants due to the risks associated with corrosion of the modular neck joint of the implant. For more information, check out the “Top 10 Things all Stryker Hip Implant Patients Need To Know”
 According to a study published in the Journal of Bone and Joint Surgery, 48 percent of the total hip arthroplasties measured in the study resulted in elevated metal ion levels for the patients that received them. The study also indicated that the rate of revision surgery administered to hip implant patients was 28 percent. We believe those numbers will continue to increase in the months and years ahead based on our own specific findings.
According to a study published in the Journal of Bone and Joint Surgery, 48 percent of the total hip arthroplasties measured in the study resulted in elevated metal ion levels for the patients that received them. The study also indicated that the rate of revision surgery administered to hip implant patients was 28 percent. We believe those numbers will continue to increase in the months and years ahead based on our own specific findings.
Those patients in the study who received a modular hip implant had the Kaplan-Meier survivorship (a measure of the life cycle of a product) of their devices measured at 40 percent after 4 years even though they are intended (and marketed to patients) to last for at least 15 years.
It comes as no surprise to us that results from this new study are consistent with what our clients have been alleging all along: The Stryker Rejuvenate or ABG II are defective and should have never been placed on the market. Our national hip implant attorneys are currently investigating Stryker hip implant recall claims on behalf of all persons who have received a Rejuvenate or ABG II modular-neck stem implant. This includes those that have already had a revision (replacement) of the hip device as well as those that have yet to have the recalled implant replaced.
If you or a loved one has suffered medical complications from the Stryker Rejuvenate or ABG II, contact the national personal injury lawyers at Childers, Schlueter & Smith LLC for a thorough investigation into your claim. All inquiries are kept confidential and all initial consultations are free of charge.
Other Stryker Hip Implants News
Failed Stryker hip implants have caused metal poisoning, tissue damage, and painful revision surgeries. Learn the top 10 things every patient needs to know—and how to protect your legal rights.
Hundreds of Stryker LFIT V40 hip implant cases have now been consolidated in federal court, with bellwether trials on the horizon. Here’s the latest update on the litigation.
A federal judge has allowed key claims to proceed in a Stryker Gamma3 hip implant lawsuit, including those related to manufacturing defects and breach of warranty.
The New Jersey Supreme Court has approved the consolidation of state court lawsuits involving Stryker’s LFIT Anatomic Cobalt Chromium V40 femoral heads into multicounty litigation.
Recent lawsuit filings over Stryker’s Accolade V40 combination hip implants suggest widespread failure risks—yet most patients don’t even know they may be affected.
Although some Stryker LFIT V40 femoral heads were recalled in 2016, emerging cases reveal corrosion and failure risks in other models—especially when paired with the Accolade TMZF stem.